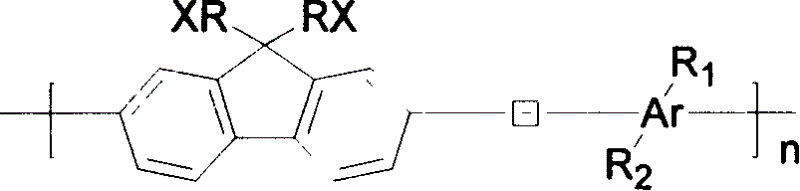
Fluorene based water soluble conjugated polymer and process for preparing same
A compound and group technology, applied in biological testing, material testing products, organic chemistry, etc., can solve the problems of poor polymer stability, low detection sensitivity, weak luminescence intensity, etc., and achieve excellent luminescence performance, thermal stability, etc. The effect of good performance and excellent luminous properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
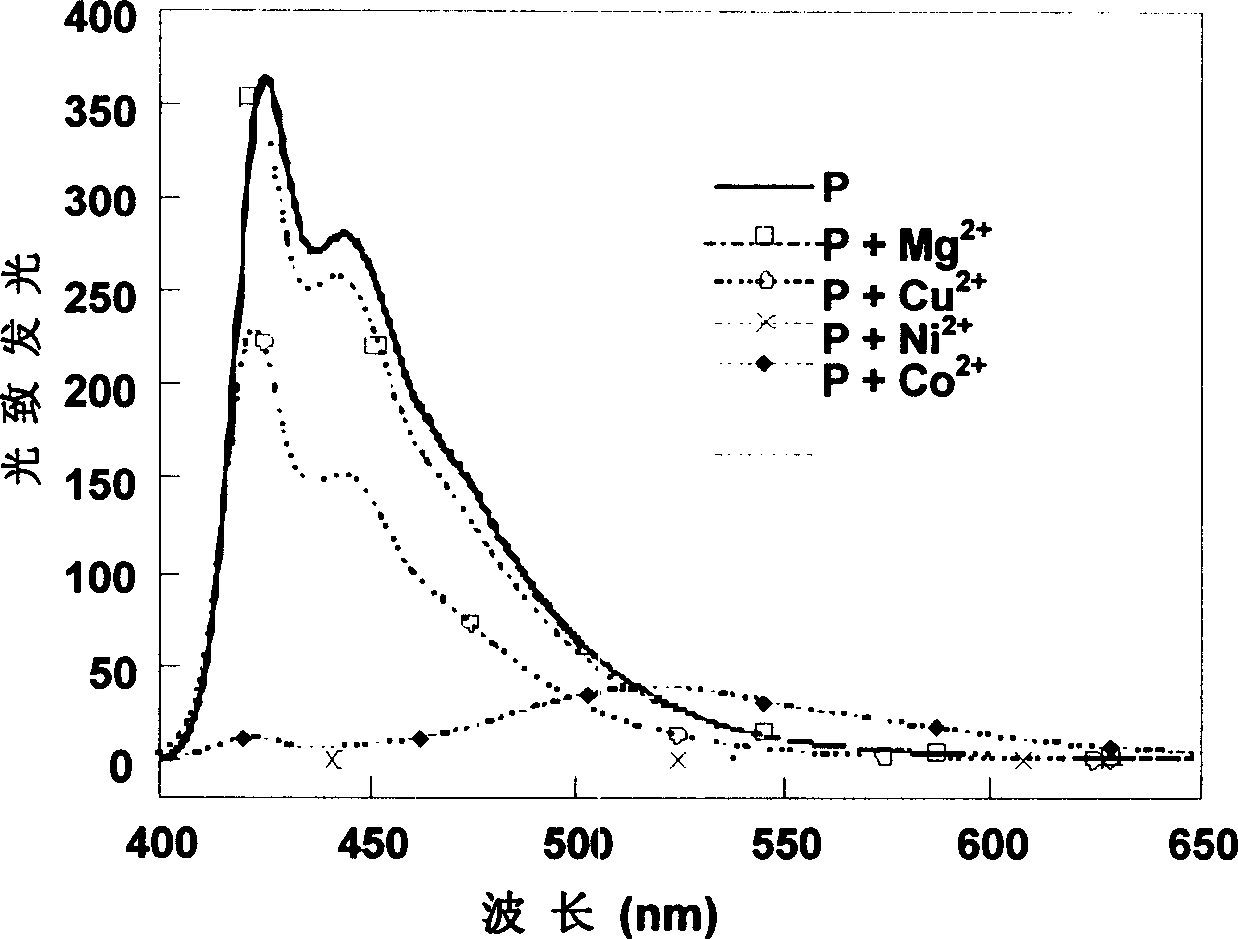
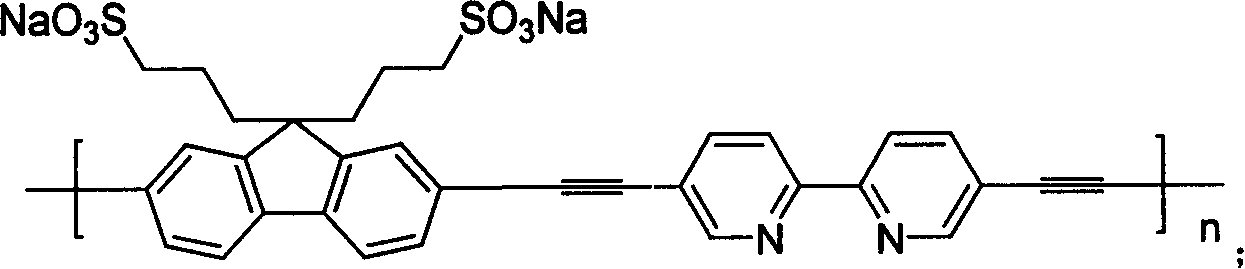
[0056] Embodiment 1, the synthesis of Poly[9,9-di(3-sulfonatopropyl)fluorene-2,7-yleneethylene-co-alt-5,5'-(2,2'-bipyridylene)]
[0057] 2, 7-dibromofluorene and 3-bromopropanol, 50% NaOH and the catalyst were refluxed in DMSO for 12 hours, and CH 2 Cl 2 Extract and spin dry the solvent to obtain crude product. The crude product was separated by column chromatography to obtain 2,7-dibromo-9,9-di(3-hydroxyl-propyl)-fluorene (1) as a yellow powder solid. The intermediate (1) was reacted with MsCl in a mixed solution of THF and triethylamine for 3 hours and filtered, and the filtrate was spin-dried to obtain a yellow solid crude product. The crude product was separated by column chromatography to obtain 2,7-dibromo-9,9-di(3-methane-sulfonatopropyl)-fluorene (2) as a yellow powdery solid. Intermediate (2) was reacted with NaI in acetone solution for 24 hours and filtered, and the crude product was separated by column chromatography to obtain light yellow crystal 2,7-dibromo-9,9...
Embodiment 2
[0062] Embodiment 2, the synthesis of Poly[(9,9-di(3-sulfonatopropylfluorene-col-alt-(1,4-benzo-18-crown-6)]
[0063] Add compound 4, 1, 4-bromo-benzo-18-crown-6 and catalyst [Pd(PPh 3 ) 4 ], CuI. The reaction system was heated to 70°C and maintained for 24 hours. After the reaction was completed, it was cooled to room temperature, the reaction solution was dropped into methanol, and the resulting precipitate was collected. After the precipitate was washed with methanol, the reaction was washed with acetone in a Soxhlet extractor for 24 hours to remove oligomers and excess catalyst, and after vacuum drying, a bright yellow solid was obtained
[0064] Poly[(9,9-di(3-sulfonatopropylfluorene-col-alt-(1,4-benzo-18-crown-6))].
[0065] 2. Cationic water-soluble polymer:
Embodiment 3
[0066] Example 3, Poly[(9,9-bis(3'-((N,N-dimethyl)-N-ethylammonium)-propyl)-2,7-fluorene)-co-alt-5,5'-( Synthesis of 2,2'-bipyridylethylene)]Dibromide
[0067] 2,7-dibromofluorene and 3-dimethylaminopropylchloride hydrochloride, 50% NaOH and the catalyst were refluxed in DMSO for 12 hours, and then the 2 Cl 2 Extract and spin dry the solvent to obtain crude product. The crude product was recrystallized to obtain white crystals of 2,7-dibromo-9,9-bis(3'-(N,N-dimethylamino)propyl)-fluorene(1')
[0068] With intermediate (1 '), compound 7 in embodiment 1, catalyst [Pd(PPh 3 ) 4 ], CuI mixed and dissolved in toluene. Under the protection of inert gas, the reaction system was heated to 70° C., and the reaction was maintained for 72 hours. After the reaction was completed, it was cooled to room temperature, the reaction solution was dropped into methanol, and the resulting precipitate was collected. After the precipitate was washed with methanol, the reaction was washed with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com