Human parathyrin 1.34 peptide related peptide-Pro-Pro-[Arg11 hPTH (1.34)-Pro-Pro
A technology for parathyroid hormone and related peptides, which is applied in DNA recombination technology and medical related fields, and can solve problems such as limitation of action of carboxypeptidase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0131] gene synthesis
[0132] 1. According to Pro-Pro-[Arg 11 The amino acid sequence of ]-hPTH(1-34)-Pro-Pro peptide was selected from Escherichia coli preferred codons, designed and synthesized by Shanghai Sangon Bioengineering Technology Service Co., Ltd. on the 391 type DNA automatic synthesizer of PE Company in the United States as follows 3 fragments.
[0133] (1) 5′-GGG GGA TCC ACC GTC CGT TTC CGA AAT CCA ACT GAT GCA TAA TCGTGG TAAACA TCA G-3′
[0134](2) 5′-AAAAAG CTT ACG GCG GGAAGT TAT GTA CAT CCT GCA GTT TC-3′
[0135] (3) 5′-TGCCAAGCTTACGGCGGG-3′
[0136] 2. PCR amplification to obtain the target gene. DNA sequence analysis was determined by Shanghai Bioengineering Company. The sequencing results confirmed that the sequence of the PCR product obtained by using the artificially synthesized gene fragments (1) and (2) as primers and the pED-hPTH(1-34) recombinant plasmid as a template contained the nucleotide sequence shown in SEQ ID NO: 1 acid sequence.
[013...
Embodiment 2
[0139] Construction of engineered bacteria
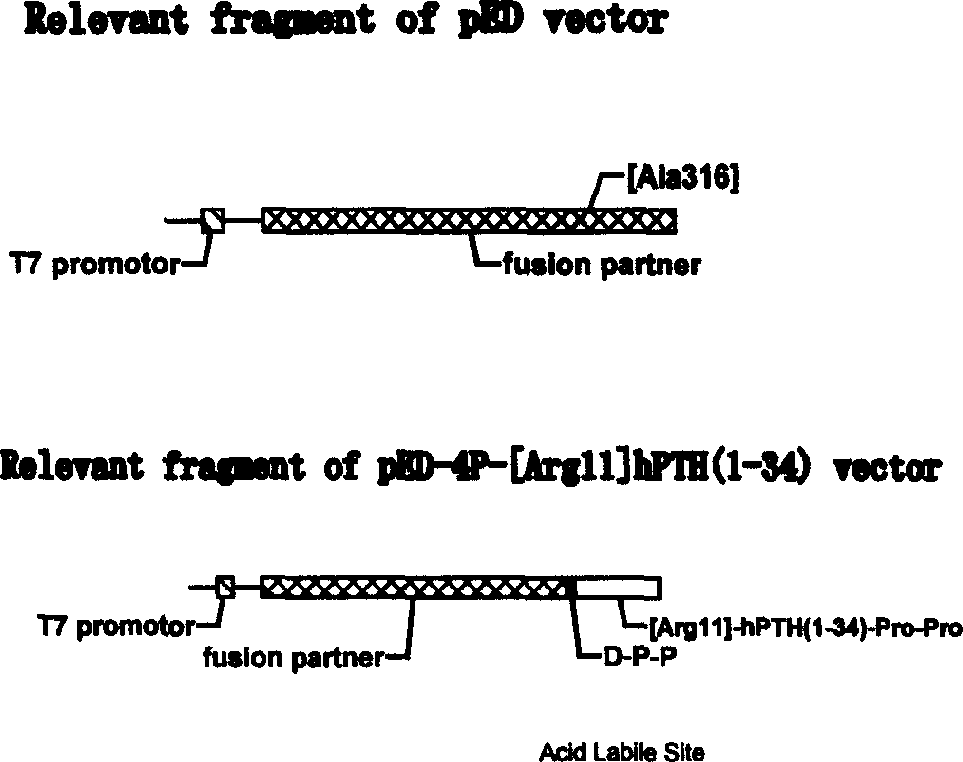
[0140] pED is a recombinant plasmid suitable for high-efficiency expression and processing of short peptides constructed by our laboratory. This recombinant plasmid will eliminate the only acid hydrolysis site of L-asparaginase C-terminal 127 peptide gene and a set of multiple cloning sites It was constructed between the NcoI and BamHI sites of the introduced plasmid pET28a.
[0141] (1) Use restriction endonucleases BamHI and HindIII to cut out the hPTH (1-34) gene synthesized by artificial and PCR methods and insert it between the corresponding sites of pED, and keep the original reading frame. Recombinant plasmid The recombinant plasmid pED-hPTH(1-34) was screened out by restriction enzyme analysis. Transformation of competent BL21 (DE3) to obtain the corresponding engineering bacteria, DNA sequence analysis is also in line with expectations. The strain was named Escherichia coli pED-hPTH(1-34) / BL21.
[0142] (2) The target ge...
Embodiment 3
[0144] Fermentation of genetically engineered bacteria and Pro-Pro-[Arg 11 Expression and Purification of Fusion Protein of ]-hPTH(1-34)-Pro-Pro Peptide
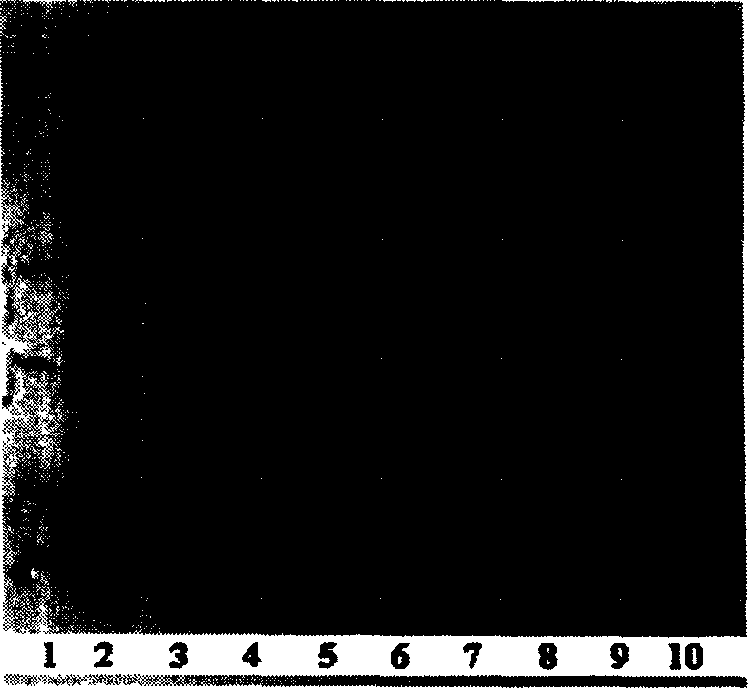
[0145] The monoclonal engineering bacteria pED-4P-[Arg 11 ]hPTH(1-34) / BL21 was inoculated with liquid LB medium containing kanamycin, cultured with shaking at 37°C until the A600 was about 0.6, and the inducer lactose was added to a final concentration of 5 mmol. After continuing to culture for 7 hours, samples were taken for SDS-polyacrylamide gel electrophoresis analysis, and a new protein band appeared at a molecular weight of about 22000D (Figure 1B), which was consistent with the theoretically estimated value. Densitometric analysis analysis showed that: after induction for 7h, pED-4P-[Arg 11 ] The fusion protein expressed by hPTH(1-34) / BL21 accounted for 53.6% of the total protein of the bacteria, and the inclusion body was formed in the cell. Precipitate the fusion protein in 4M urea with 0.68V ethanol, pre-cool at...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com