Gonadotrophins for folliculogenesis
A follicle and superovulation technology, applied in the field of gonadotropins, can solve problems such as limiting the number of embryos
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
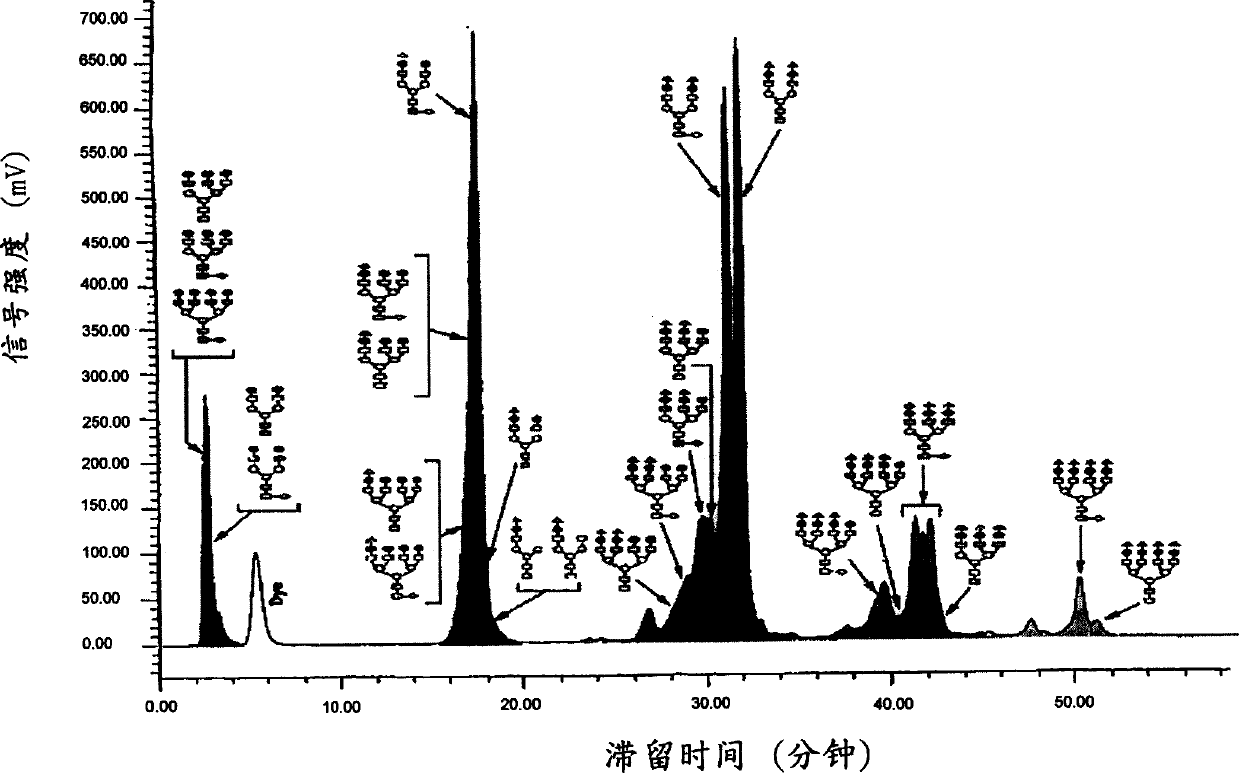
[0101] The mensuration of embodiment 1 Z numerical value
[0102] Based on the glycan profile, the Z value of the glycoprotein can be determined.
[0103] Recombinant human FSH was reacted with hydrazine at 100°C for 5 hours, and glycan moieties were released using an Oxford GlycoSciences GlycoPrep(R) 1000 automatic analyzer or equivalent.
[0104] Glycans are separated from unreacted hydrazine and amino acid hydrazides using a glass bead-coated column. Glycans were eluted with sodium acetate reagent.
[0105] Glycans are acetylated with acetic anhydride. Excess reagents were removed using a mixed bed ion exchange column. Any unreduced glycans were collected in dilute acetate buffer.
[0106] Glycans were collected on 0.5 meter filters (Oxford GlycoSciences) and lyophilized. Under acidic conditions, the dry polysaccharides are reacted with a reducing agent with a fluorophore (such as 2-aminobenzamide) at 65° C. for 120 minutes to label the dry polysaccharides.
[0107] T...
Embodiment 2
[0124] Embodiment 2 Measurement of Antenna Index (AI)
[0125] Glycans were released from the peptide backbone by hydrazinolysis as described in Example 1, and these carbohydrates were then fluorescently labeled with 2-aminobenzamide.
[0126] 2-Aminobenzamide-labeled glycans were enzymatically desialylated with sialidase (Vibrio cholerae) in 250 mM ammonium acetate pH 5.5 containing 20 mM calcium chloride for 18 hours at 37°C. A starting amount of glycans of 100 μg rhFSH uses about 0.05 U sialidase.
[0127] The desialylated glycans were vacuum-dried and stored at -20°C until separation by preparative reverse-phase HPLC under the following conditions:
[0128] The column is a GlycoSep® R column;
[0129] The flow rate of the mobile phase is 0.7 ml / min;
[0130] Eluent A: Ammonium acetate 50mM pH6.0
[0131] Eluent B: Ammonium acetate 50mM pH6.0 with 8% acetonitrile
[0132] Detect with a fluorometer and set λ 激发 : 330nm, λ 发射 : 420nm;
[0133] • Column temperature: 30...
Embodiment 3
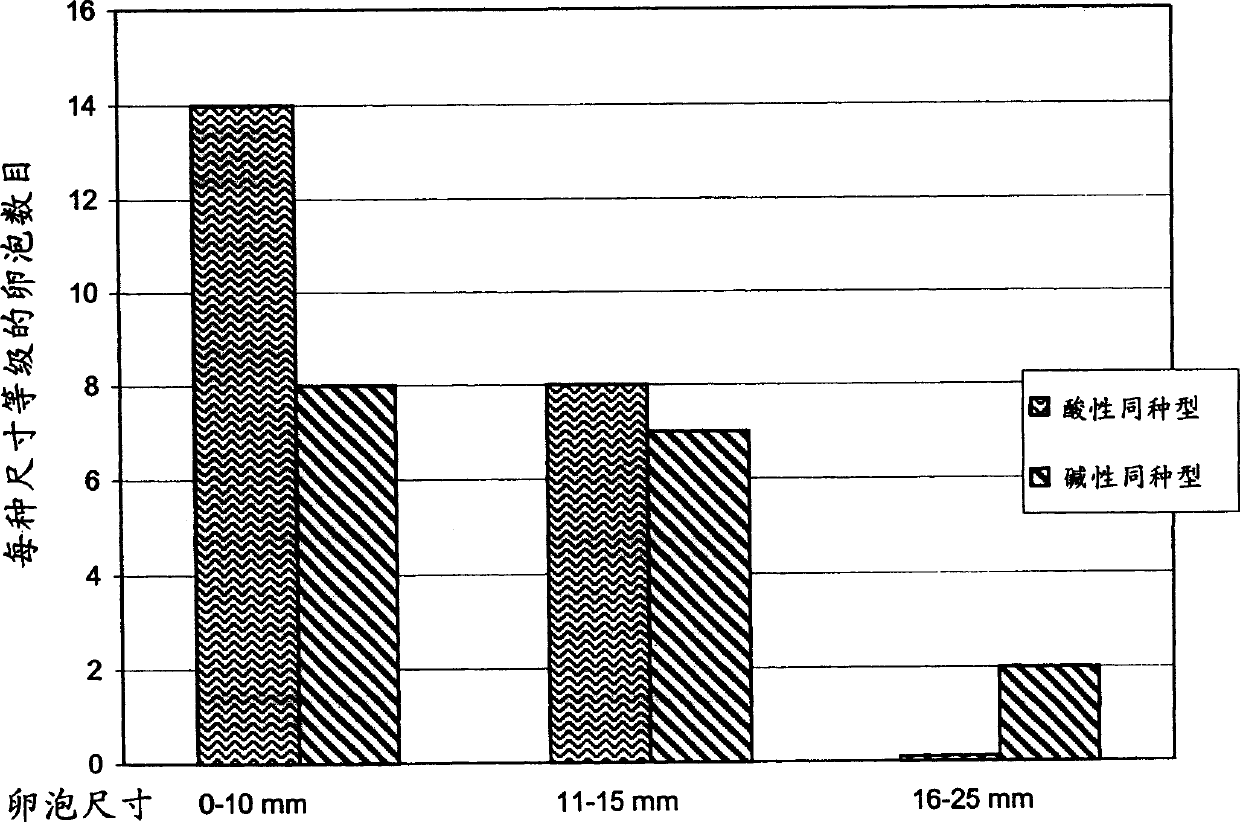
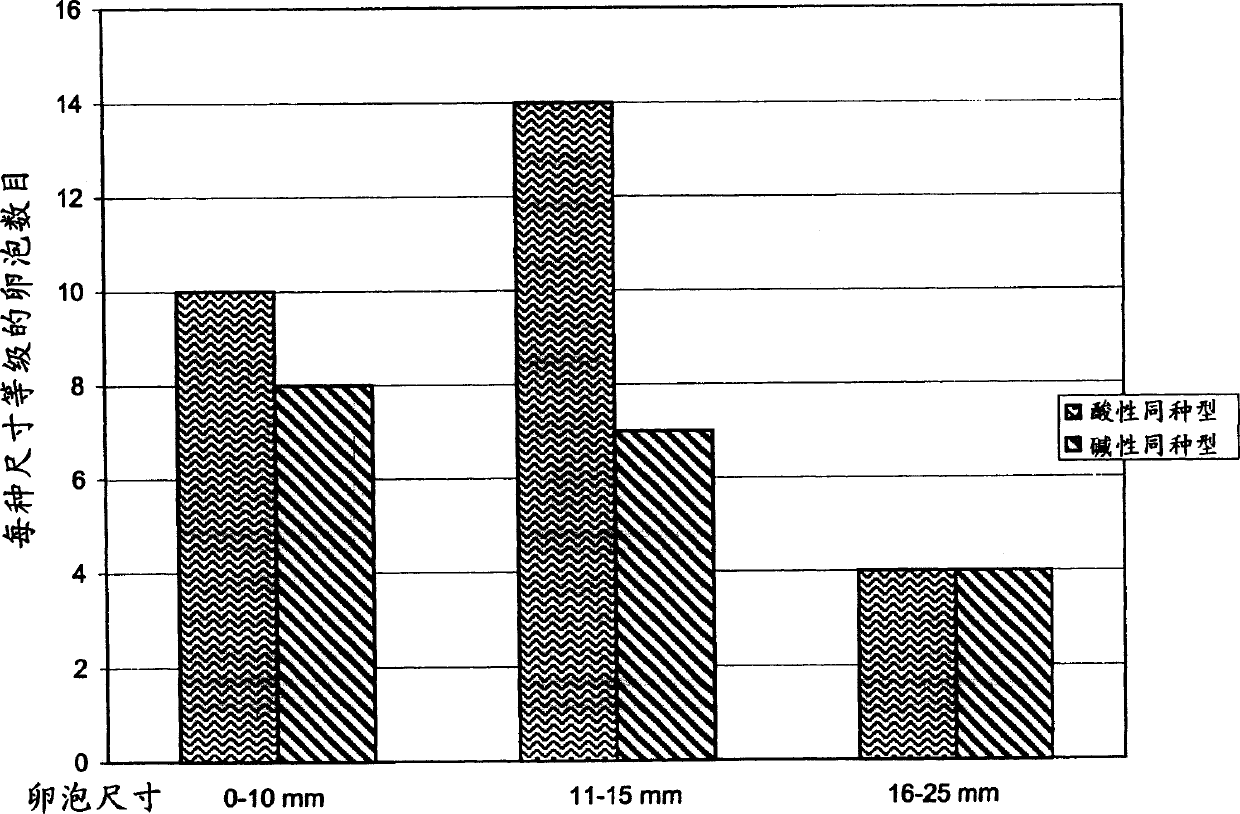
[0145] Example 3 FSH is separated into different components based on the degree of sialylation
[0146] Recombinant FSH was separated into acidic and basic components by anion exchange chromatography on DEAE Sepharose FF.
[0147] The column used was packed with DEAE Sepharose FF resin: Φ 1.6 x 20 cm (XK Pharmacia or equivalent) for experimental grade purity (about 60 mg whole protein), Φ 3 for larger scale purity .4 x 40 cm (Vantadge Amicon or whatever);
[0148] The flow rate of the mobile phase is 150-250 cm / hour;
[0149] Equilibrium buffer 1: 2M Tris-HCl pH7.0±0.1;
[0150] Equilibrium buffer 2: 25mM Tris-HCl pH7.0±0.1, conductivity is 2.15±1.5mS / cm;
[0151] Elution buffer 1: 25mM Tris pH 7.0±0.1, 35mM NaCl, conductivity 5.8±0.4mS / cm (this buffer elutes the more basic isoform);
[0152] Elution buffer 2: 25mM Tris pH 7.0±0.1, 150mM NaCl, conductivity 18.3±0.5mS / cm (this buffer elutes the more acidic isoform);
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com