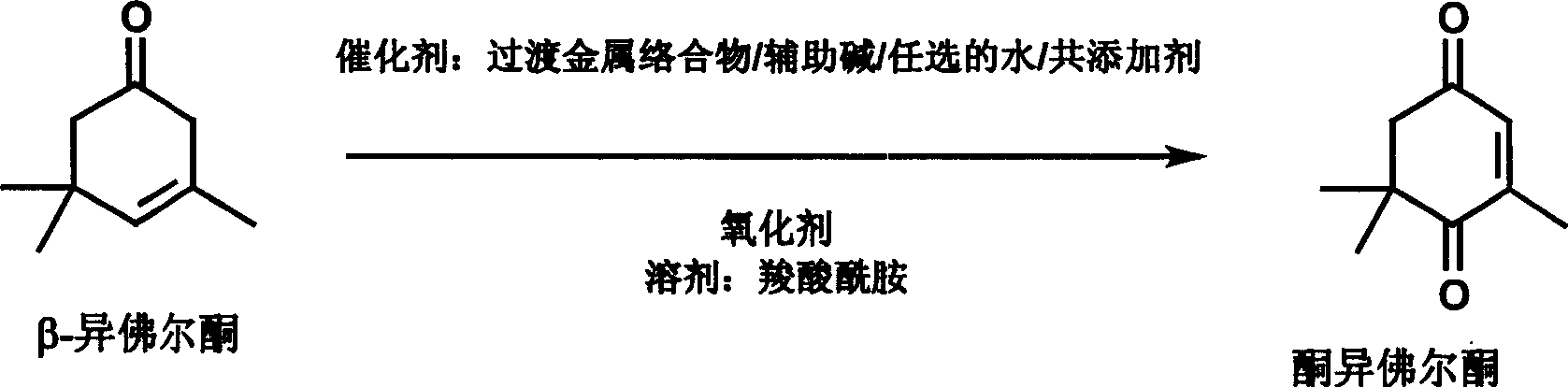
Preparation of 3, 5, 5-trimethyl-cyclohex-2-ene-1, 4-dione
A technology of trimethyl and cyclohexane is applied in the field of preparation of 3,5,5-trimethyl-cyclohex-2-ene-1,4-dione, and can solve problems such as adverse effects of preparation cost and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-12
[0072] First, 58.0 g of solvent specified in Table 1, 1.2 g of water, 0.16 g of acetylacetone, 2.53 g of triethylamine and the amount of Saren manganese that can be seen from Table 1 were put into a glass beaker and stirred for 15 minutes (variation variant A) or 16 hours (variant B). Then 15.4 g of β-IP were added and the reaction mixture was briefly stirred and transferred into a bubble column. Under normal pressure and 35°C, it was blown with oxygen (12 l / h) for 2.5 hours, and then the yield of KIP was determined by gas chromatography with internal standard method. The results are listed in Table 1.
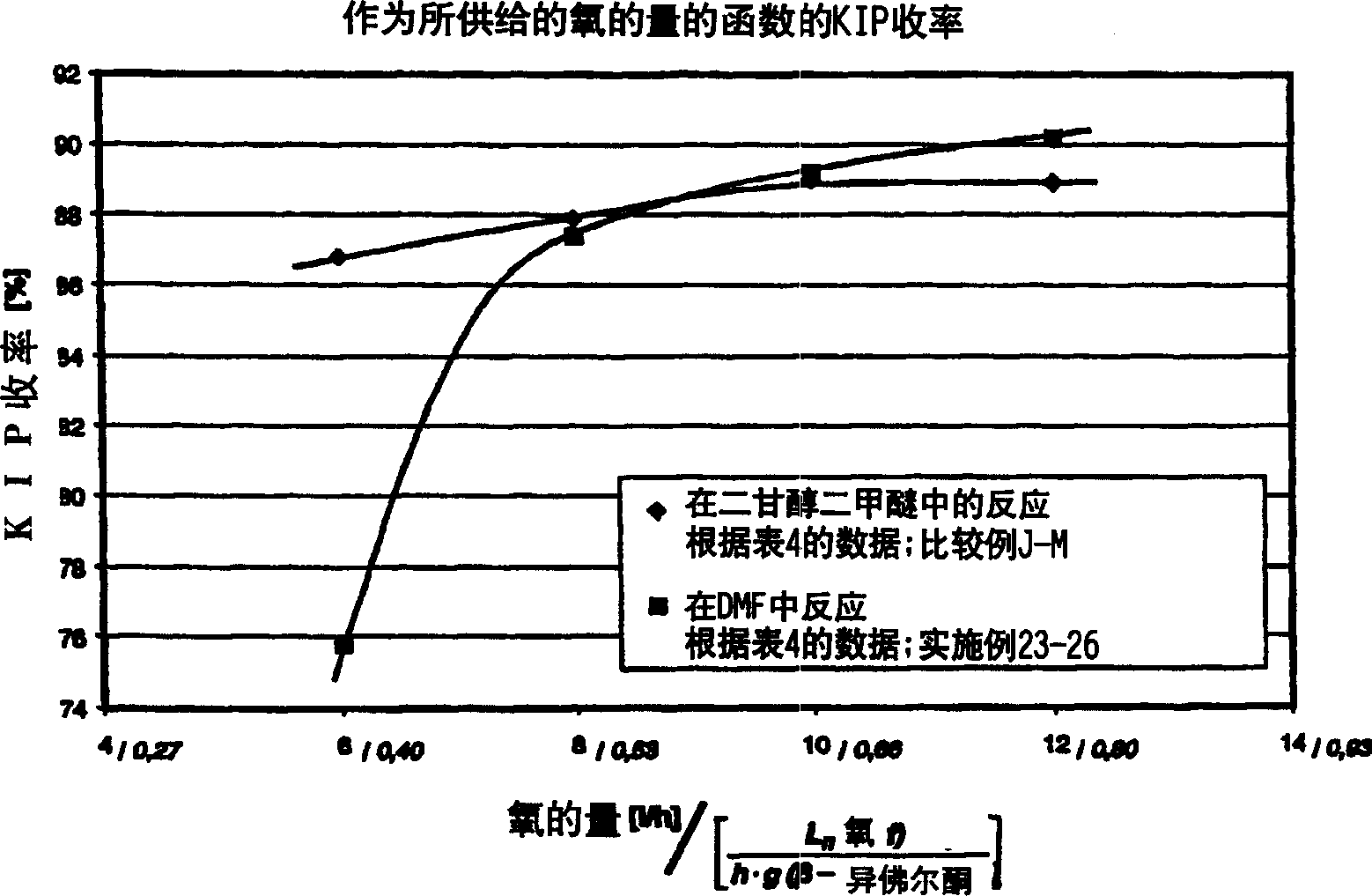
Embodiment 13-15
[0077] First 72.5 grams of DMF, 1.56 grams of water, 0.21 grams of acetylacetone, 3.18 grams of triethylamine, the amount of acid specified in Table 2, and 75 mg of Saren manganese were placed in a glass beaker and stirred for 15 minutes. Then 19.25 g of β-IP were added and the reaction mixture was briefly stirred and transferred into a bubble column. Under normal pressure and 35°C, it was blown with oxygen (16 l / h) for 2.5 hours, and then the yield of KIP was determined by gas chromatography with internal standard method. The results are listed in Table 2.
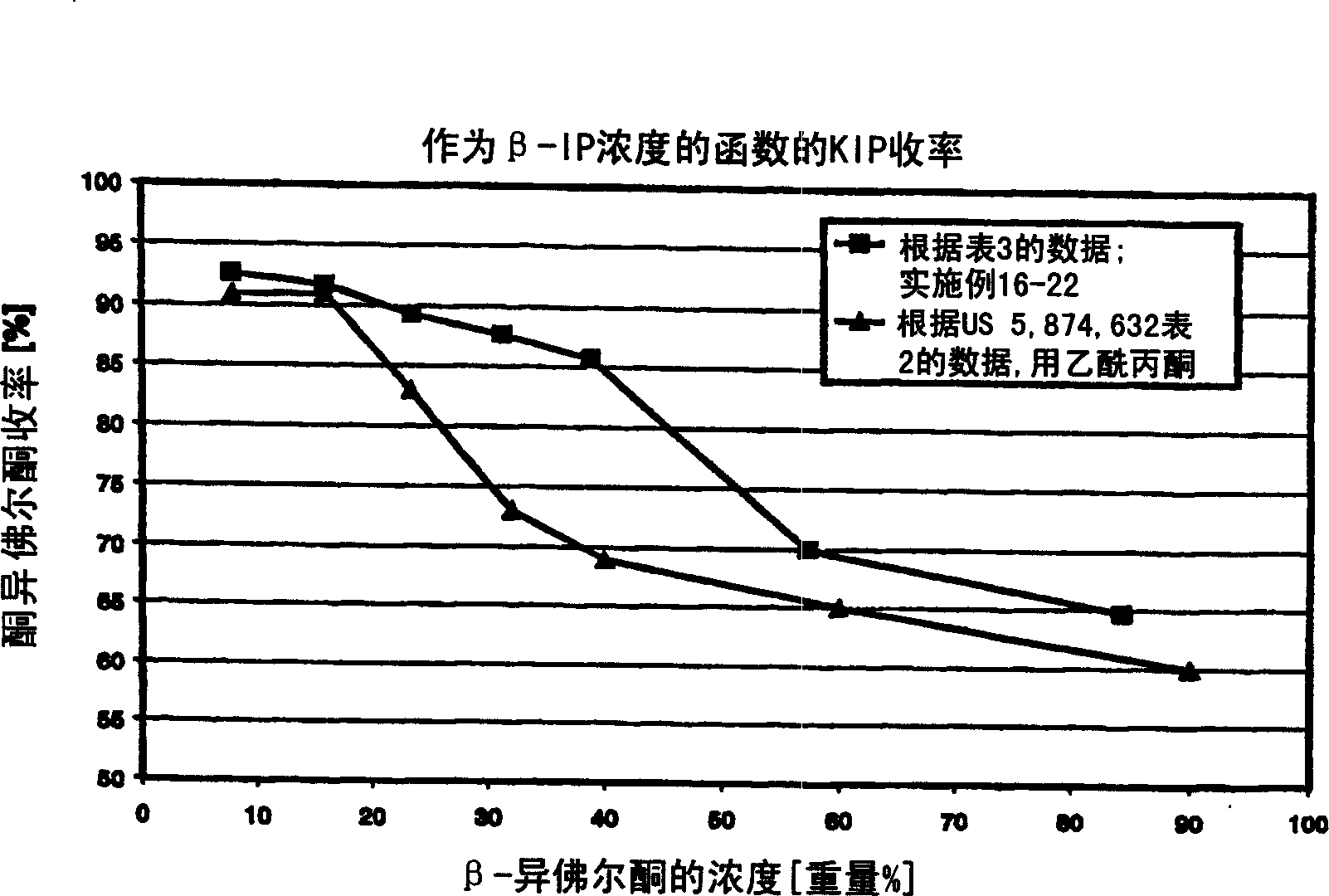
Embodiment 16-22
[0083] In a solution of approximately 100 g of β-IP, triethylamine, water, acetylacetone and saran manganese in DMF at atmospheric pressure and 35 °C, the concentration can be read from Table 3. Oxygen aeration (16 l / h) for 2.5 hours, and then the yield of KIP was determined by gas chromatography using an internal standard method. The results are listed in Table 3.
[0084] Example
β-IP
[mol / l]
[mol / l]
water
[mol / l]
[mol / l]
Salem MN
[mol / l]
KIP yield
[%]
16
0.54
0.30
0.80
115
3.81
92.6
17
1.08
0.30
0.81
113
3.79
91.7
18
1.59
0.30
0.81
167
5.57
89.4
19
2.11
0.29
0.79
222
7.38
87.7
20
2.62
0.29
0.78
273
9.14
85.7
21
3.85
0.29
0.78
37...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com