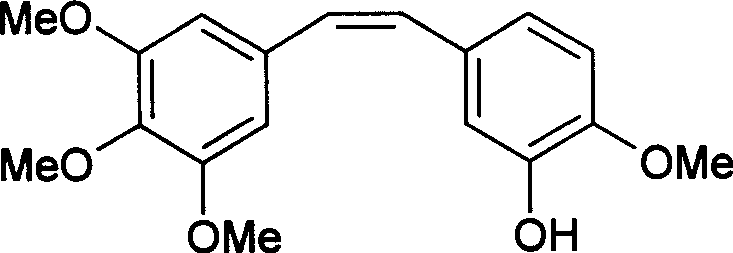
Process for preparing (z)-3'-hydroxy-3, 4, 4', 5-tetranetgixy diphenyl ethylene
A technology of tetramethoxystilbene and methoxyphenyl, which is applied in the field of preparation of -3'-hydroxyl-3, can solve the problems of difficult to buy, expensive, and expensive isovanillin, and achieve cis-trans Improved selectivity, less environmental pollution, and reduced reaction costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
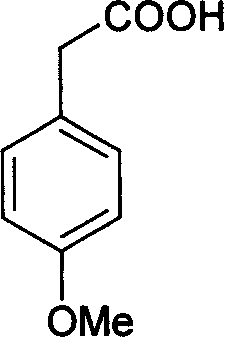
[0036] Take 10.2g (0.061mol) of p-methoxyphenylacetic acid in a three-necked flask, add 20mL of glacial acetic acid to dissolve it, then add 3.6mL of bromine (11.2g, 0.068mol) dropwise, drop it for 45 minutes, and then stir in an ice bath After 1 hour, it was poured into ice water, and the solid was precipitated, filtered, and dried to obtain 14.8 g of 3-bromo-4-methoxy-phenylacetic acid, with a yield of 98.3%, and recrystallized with ethanol-water to obtain white flaky crystals. The yield is 70%, and the melting point is 113-114°C (literature value is 114-115°C).
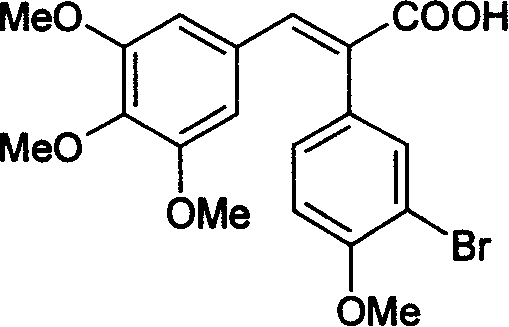
[0037]Weigh 4.92g (0.02mol) of the obtained 3-bromo-4-methoxyphenylacetic acid, and add 4.32g (0.022mol) of 3,4,5-trimethoxybenzaldehyde into the reaction flask, and use 20mL Acetic anhydride was dissolved, and 5.0 mL of triethylamine was added dropwise, heated to 130°C, and reacted for 5 hours. After acidification with concentrated hydrochloric acid, it was poured into ice water to precipitate a solid, and the ob...
Embodiment 2
[0040] Embodiment 2: the compound melting point that embodiment 1 obtains and spectral data determination
[0041] The measurement results are as follows: mp116-117°C (literature value 115-116°C). Ms m / z(%): 316(M + , 100), 301(75). 1 H NMR (δ, ppm, J / Hz): 6.91 (d, 1H, J=2.0); 6.79 (dd, 1H, J=8, 2.0); 6.71 (d, 1H, J=8.0); 6.51 (s , 2H); 6.45(d, 1H, J=12.4); 6.42(d, 1H, J=12.4); 5.49(s, 1H); 3.89(s, 3H); 3.84(s, 3H); 3.68(s , 6H). IR (KBr): 3424, 3002, 2938, 2836, 1610, 1579, 1508, 1459, 1419, 1328, 1182, 1025, 1004, 944, 881, 854, 796, 765. From the above results, it was proved that the compound was (Z)-3'-hydroxy-3,4,4',5-tetramethoxystilbene.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com