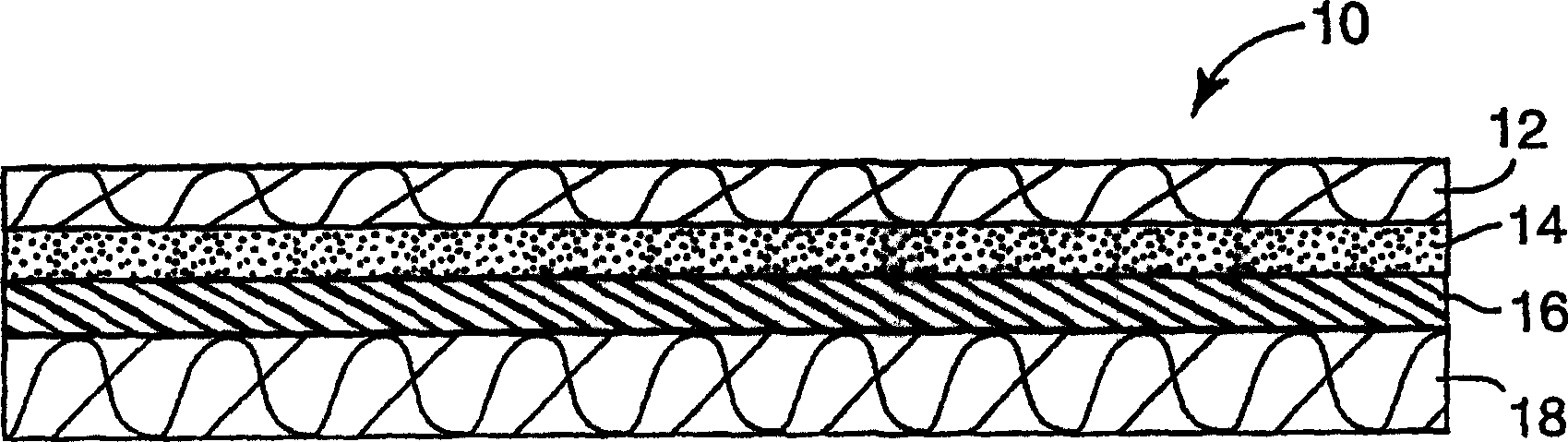
Process for preparing a k-type polarizer
A technology of polarizers and products, applied in chemical instruments and methods, polarizing elements, instruments, etc., can solve the problems of polarizers with stripes or spots, unsuitable for precision optical applications, uneven catalytic dehydration, etc., to reduce potential dangers. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-8
[0089] A series of poly(isooctyl acrylate-co-acrylic acid) polymers were prepared by varying the ratio of isooctyl acrylate (IOA) to acrylic acid (AA). Prepare 80-100 parts by weight of IOA (available from Aldrich, Milwaukee, WI), 0-20 parts by weight of AA (available from Aldrich, Milwaukee, WI), 0.4 parts by weight of 2,2'-azobis(2-methyl Nitrile) (available as VAZO 67 from DuPont, Wilmington, DE) and 150 parts by weight of ethyl acetate (available from EM Science, Gibbstown, NJ) and heated at 65°C for 24 hours. To each of the obtained polymer solutions was added 0.2 parts by weight of 2,4-bis(trichloromethyl)-6-(3,4-dimethoxyphenyl)-s-triazine as a photoacid generator. Combination of aryl nitriles with tris Chloroacetonitrile and Lewis acids such as AlCl 3 , AlBr 3 etc. co-trimerization to prepare s-triazine.
[0090]The polymer solution containing the obtained 2,4-bis(trichloromethyl)-6-(3,4-dimethoxyphenyl)-s-triazine was applied to a wet thickness of 0.254 mm using a...
Embodiment 9
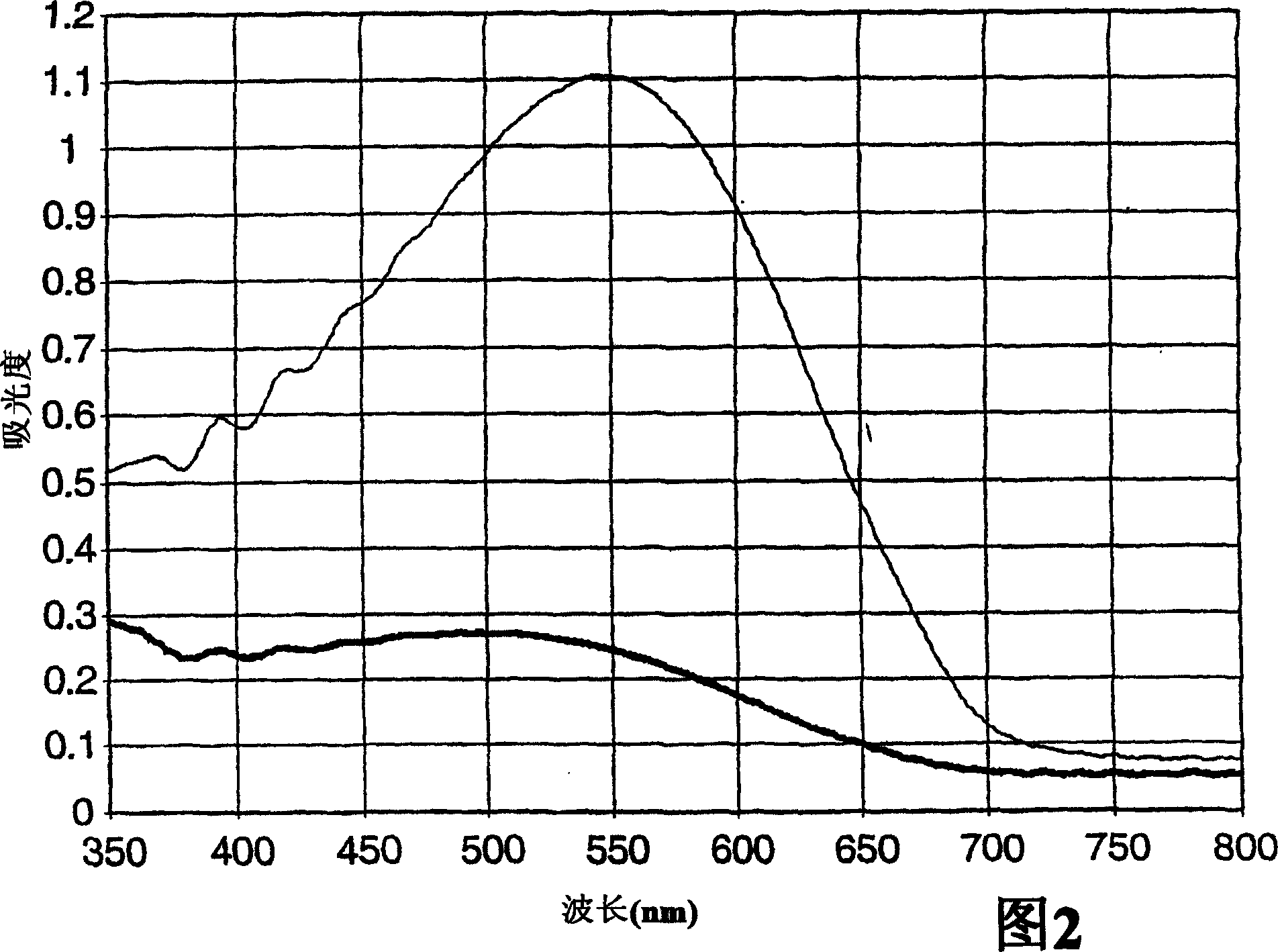
[0094] In addition to using 96 parts by weight of IOA, 4 parts by weight of AA and 0.5 parts by weight of 2,4-bis(trichloromethyl)-6-(3,4-dimethoxyphenyl)-s-triazine, according to the implementation Example 3 Preparation of a sandwich structure with a PET diffusion barrier. The resulting structures were exposed to UV light and heat as in Examples 1-8, except that the exposure time was varied. PET was removed from each exposed structure and poly(isooctyl acrylate-co-acrylic acid) was washed from the partially dehydrated PVA film with ethyl acetate. As in Examples 1-8, the resulting, exposed, partially dehydrated PVA film was analyzed for absorption spectroscopy, and determined R D . The results are shown in Table 2. A representative spectrum using sample 9L is shown in Figure 2.
[0095] sample
During UV exposure
time (minutes)
When exposed at 105°C
time (minutes)
R D
at λmax
A(=)
A(⊥)
λmax
(nano)
9A...
Embodiment 10
[0101] The resin syrup was prepared by mixing 80 parts by weight of IOA, 20 parts by weight of isobornyl acrylate (available from Aldrich, Milwaukee, WI) and 0.2 parts by weight of benzil dimethyl ketal (available as Esacure™ KB-1 from Sartomer Co., West Chester, PA), the resulting solution was sparged with nitrogen for about 15 minutes, the mixture was exposed to UV light (two F40 / 350BL fluorescent tubes) until the viscosity reached 2000-3000 cps, and then quenched with oxygen. The obtained syrup (100 parts by weight) was mixed with 0.2 parts by weight of 2,4-bis(trichloromethyl)-6-(3,4-dimethoxyphenyl)-s-triazine. The resulting solution was coated onto a PVA film at a nominal thickness of 0.127 mm to form a sandwich and exposed to UV light for 3 minutes followed by heat for 45 minutes as in Example 9. The maroon character of the polyacetylene chromophore was formed, indicating the formation of a polarizer film.
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com