Extractive from decoction of rehmannia including six elements, combination of medication, and medical use
A technology of Liuwei Dihuang Decoction and its extract, which is applied in the direction of drug combination, medical formula, medical preparations containing active ingredients, etc., can solve the problem of inability to guarantee the effectiveness of clinical medication, unclear active ingredients, and difficulty in ensuring stable and reliable product quality. Control and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
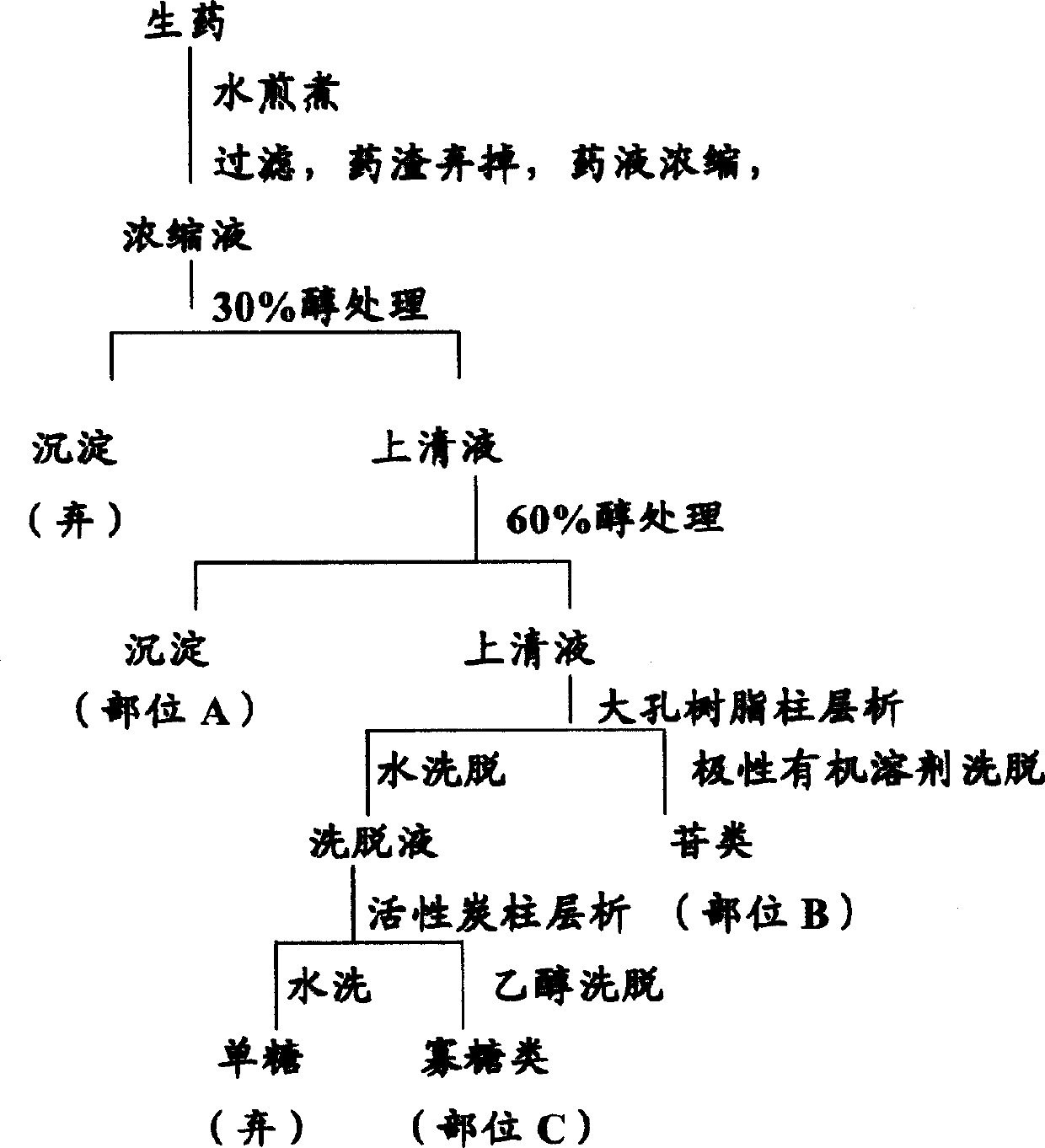
[0154] Embodiment 1: Preparation of Liuwei Dihuang Decoction Extract LW-ABC
[0155] According to the ratio of the single ingredients of Liuwei Dihuang Decoction, that is, Rehmannia glutinosa: Cornus officinalis: Chinese yam: Alisma: Alisma: Cortex paeonol: Poria (8:4:4:3:3:3) or after adding or subtracting some of the herbs, A total of 1500 grams of crude drugs are added, boiled twice in boiling water with six times the weight of crude drugs, each time for two hours, filtered with gauze (six layers) and absorbent cotton, concentrated under reduced pressure until the ratio of crude drug (weight) to extract (volume) is 1: 1.
[0156] Add ethanol to the extract with stirring to a final ethanol concentration of 30% (v / v), stir, and place overnight. Centrifuge (2500 rpm, 25 min). The precipitate was washed 4 times with 30% ethanol, centrifuged, and the precipitate was discarded. The supernatants were combined and concentrated to 1000 ml, and the final ethanol concentration was ...
Embodiment 2
[0159] Embodiment 2: Experiment of biological activity of the extract LW-ABC of Liuwei Dihuang Decoction of embodiment 1
[0160] 1. The effect of LW-ABC on immune function
[0161] 1. The effect of LW-ABC on the immune function of "kidney yin deficiency" mice
[0162] The preparation of the "kidney yin deficiency" mouse model was carried out as follows: Balb / c mice were subcutaneously injected with corticosterone suspension, 25 mg / kg, 2 times / day, for 7 consecutive days. Liuwei Dihuang Tang or the active ingredient composition LW-ABC of Liuwei Dihuang Tang was given by intragastric administration on the day of the test, once a day for 7 consecutive days, and the experiment was carried out 4 hours after the last administration. The mice were decapitated after weighing, and the thymus and spleen were removed, weighed, and the index of thymus and spleen was calculated. During the lymphocyte proliferation reaction experiment, the mice were decapitated, and the spleen was taken ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com