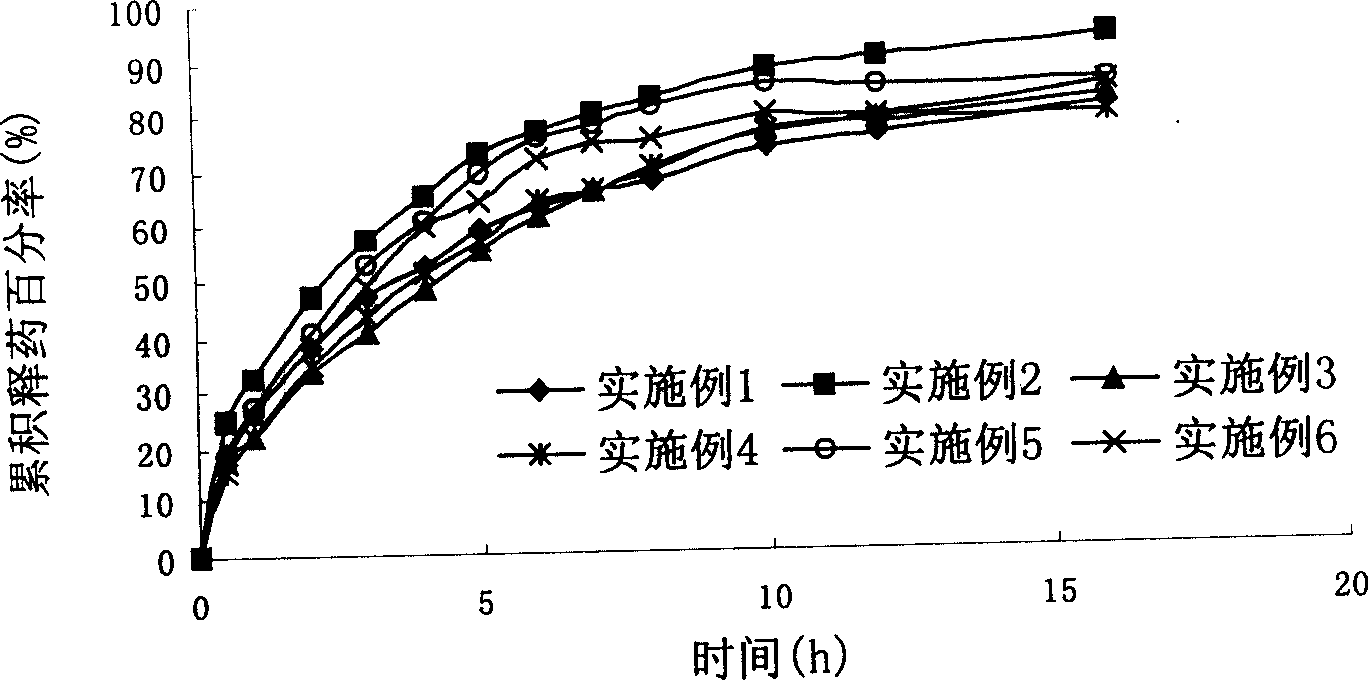
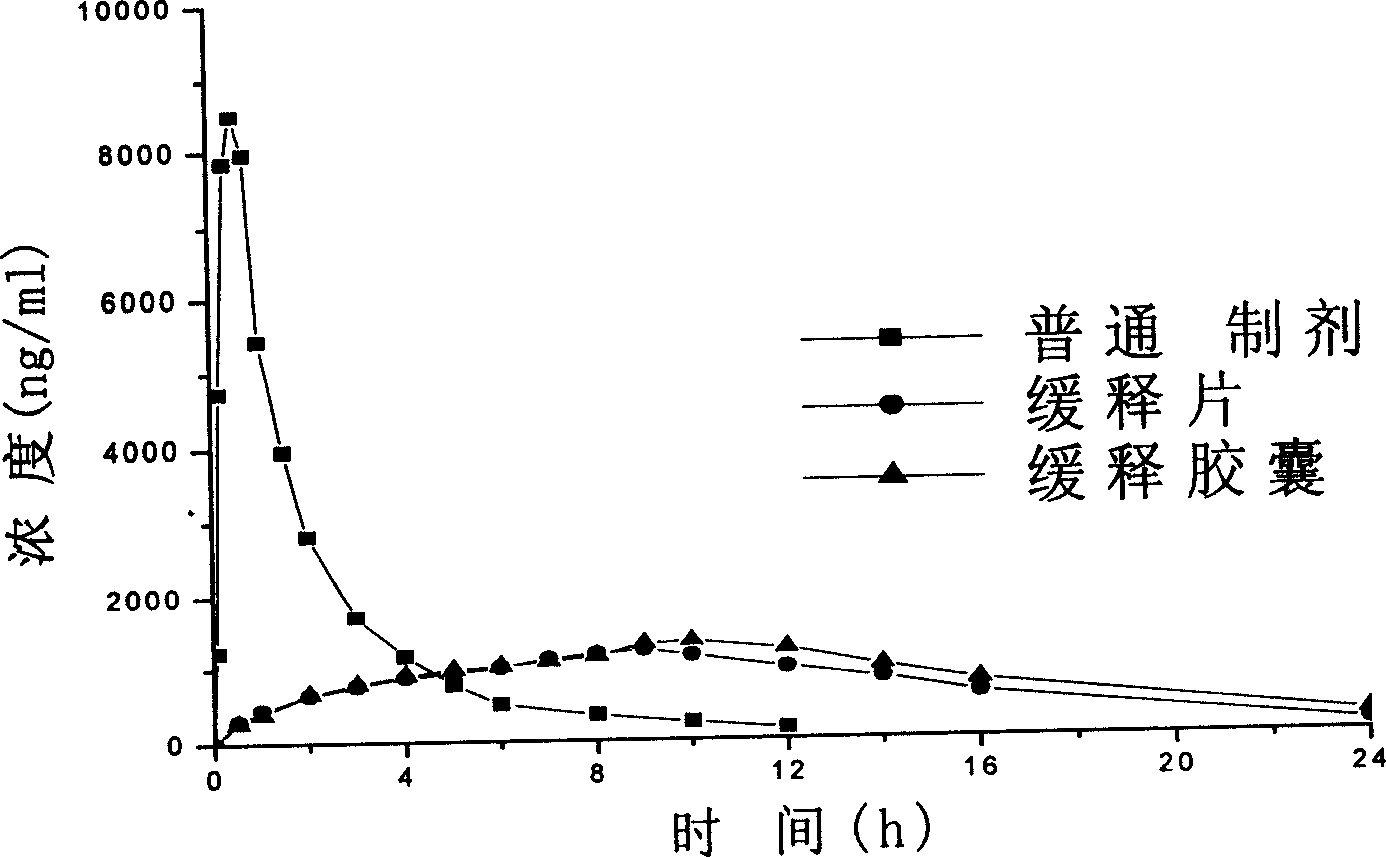
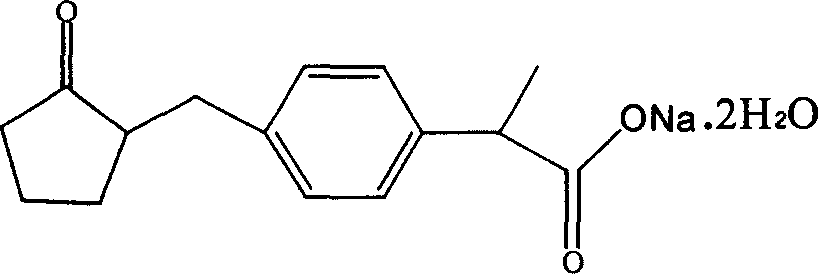
Loxoprofen sodium sustained release preparation
A technology of loxoprofen sodium and sustained-release preparations, applied in the field of medicine, can solve the problems of inability to show curative effect, peak-to-valley phenomenon, side effects and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] prescription:
[0052] Loxoprofen sodium (calculated as anhydrous substance) 180mg
[0053] Hypromellose 60mg
[0054] Ethylcellulose 160mg
[0055]Acrylic resin (Eudragit L100) 100mg
[0056] Magnesium Stearate Appropriate amount
[0057] 70% ethanol appropriate amount
[0058] Preparation method:
[0059] Loxoprofen sodium, hypromellose, ethyl cellulose and acrylic resin are crossed 80 mesh sieves respectively (if necessary, must be pulverized earlier), take each adjuvant by prescription quantity and mix homogeneously, add loxoprofen sodium, Mix evenly, add 70% ethanol to make soft material, granulate with 20-mesh sieve, dry at 50°C for 2 hours, granulate with 18-mesh sieve, add 2% magnesium stearate by dry granule weight, and press into tablets to obtain.
Embodiment 2
[0061] prescription:
[0062] Loxoprofen sodium (calculated as anhydrous substance) 180mg
[0063] Hypromellose 60mg
[0064] Ethylcellulose 60mg
[0065] Acrylic resin (Eudragit L100) 160mg
[0066] Lactose 40mg
[0067] Magnesium Stearate Appropriate amount
[0068] 70% ethanol appropriate amount
[0069] Preparation method:
[0070] Pass loxoprofen sodium, lactose, hypromellose, ethyl cellulose and acrylic resin through 80-mesh sieve respectively (if necessary, first pulverize), weigh each auxiliary material according to the prescription amount and mix evenly, add loxoprofen Sodium, mix well, add 70% ethanol to make soft material, granulate with 20-mesh sieve, dry at 50°C for 2 hours, granulate with 18-mesh sieve, add 2% magnesium stearate by weight of dry granules, compress into tablets, and obtain.
Embodiment 3
[0072] prescription:
[0073] Loxoprofen sodium (calculated as anhydrous substance) 180mg
[0074] Hypromellose 60mg
[0075] Ethylcellulose 180mg
[0076] Acrylic resin (Eudragit L100) 80mg
[0077] Magnesium Stearate Appropriate amount
[0078] 70% Alcohol Appropriate amount
[0079] Preparation method:
[0080] Loxoprofen sodium, hypromellose, ethyl cellulose and acrylic resin are crossed 80 mesh sieves respectively (if necessary, must be pulverized earlier), take each adjuvant by prescription quantity and mix homogeneously, add loxoprofen sodium, Mix evenly, add 70% ethanol to make soft material, granulate with 20-mesh sieve, dry at 50°C for 2 hours, granulate with 18-mesh sieve, add 2% magnesium stearate by dry granule weight, and press into tablets to obtain.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com