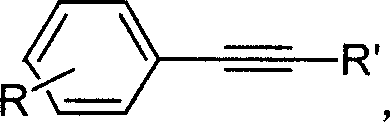
Coupling reaction of end group alkine and aryl halide
A technology for aryl halide and coupling reaction, which is applied in the field of coupling reaction of terminal alkyne and aryl halide, can solve the problems of expensive palladium catalyst, environmental pollution and serious problems, and achieves mild reaction conditions and good application. Prospect, cheap effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 1. Preparation of 1-methoxy-4-phenylethynylbenzene
[0026]
[0027] In a reaction tube, add 122mg phenylacetylene (MW=102.14, 1.2mmol), then add 234mg p-methoxyiodobenzene (MW=234.04, 1mmol), 414mg K 2 CO 3 (MW=138.21, 3mmol), 42mg N, N-dimethylglycine hydrochloride (MW=139.58, 0.3mmol), 19mg CuI (MW=190.446, 0.1mmol), 2ml DMF and 0.05ml water are as solvent, in Under argon protection, react in an oil bath at 100°C for 24 hours, cool, add 2 ml of water, extract with 4 ml of ethyl acetate each time, repeat three times, wash the extract with saturated brine, dry over anhydrous sodium sulfate, and filter. The filtrate was distilled under reduced pressure and separated through a silica gel column (petroleum ether as the eluent) to obtain 175 mg of the product 1-methoxy-4-phenylethynyl-benzene with a yield of 84%.
[0028] 1 H NMR (CDCl 3 , 300MHz) δ 3.81(s, 3H), 6.87(d, J=8.4Hz, 2H), 7.31-7.53(m, 7H); MS m / z 208(M + ).
Embodiment 2
[0030] 2. 4-Phenylethynylbenzoic acid
[0031]
[0032] In a reaction tube, add 122mg phenylacetylene (MW=102.14, 1.2mmol), then add 248mg p-iodobenzoic acid (MW=248.02, 1mmol), 414mg K 2 CO 3 (MW=138.21, 3mmol), 42mgN, N-dimethylglycine hydrochloride (MW=139.58, 0.3mmol), 19mg CuI (MW=190.446, 0.1mmol), 2ml DMF and 0.05ml water are as solvent, in argon Under air protection, react in an oil bath at 100°C for 24 hours, cool, add 2 ml of water, wash with 2N hydrochloric acid until PH = 2, extract with 4 ml of ethyl acetate each time, repeat three times, and wash the extract with saturated saline. After drying over sodium sulfate, filter, the filtrate was distilled under reduced pressure, and separated through a silica gel column (petroleum ether: ethyl acetate = 1:1 as the eluent) to obtain 153 mg of the product 4-phenylethynylbenzoic acid, with a yield of 69%.
[0033] 1 H NMR (CD 3 COCD 3 , 300MHz) δ7.45(t, J=3.3Hz, 2H), 7.59-7.62(m, 1H), 7.68(d, J=8.1Hz, 1H), 7.80(d, ...
Embodiment 3
[0035] 3. Preparation of 1-methoxy-2-phenylethynyl-benzene
[0036]
[0037] In a reaction tube, add 122mg phenylacetylene (MW=102.14, 1.2mmol), then add 234mg o-methoxy iodobenzene (MW=234.04, 1mmol), 414mg K 2 CO 3(MW=138.21, 3mmol), 42mg N, N-dimethylglycine hydrochloride (MW=139.58, 0.3mmol), 19mg CuI (MW=190.446, 0.1mmol), 2ml DMF and 0.05ml water are as solvent, in Under argon protection, react in an oil bath at 100°C for 24 hours, cool, add 2 ml of water, extract with 4 ml of ethyl acetate each time, repeat three times, wash the extract with saturated brine, dry over anhydrous sodium sulfate, and filter. The filtrate was distilled under reduced pressure and separated through a silica gel column (petroleum ether as the eluent) to obtain 172 mg of the product 1-methoxy-2-phenylethynyl-benzene with a yield of 82%.
[0038] 1 H NMR (CDCl 3 , 300MHz) δ3.91(s, 3H), 6.89-6.97(m, 2H), 7.25-7.36(m, 4H), 7.49-7.58(m, 3H); MS m / z 208(M + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com