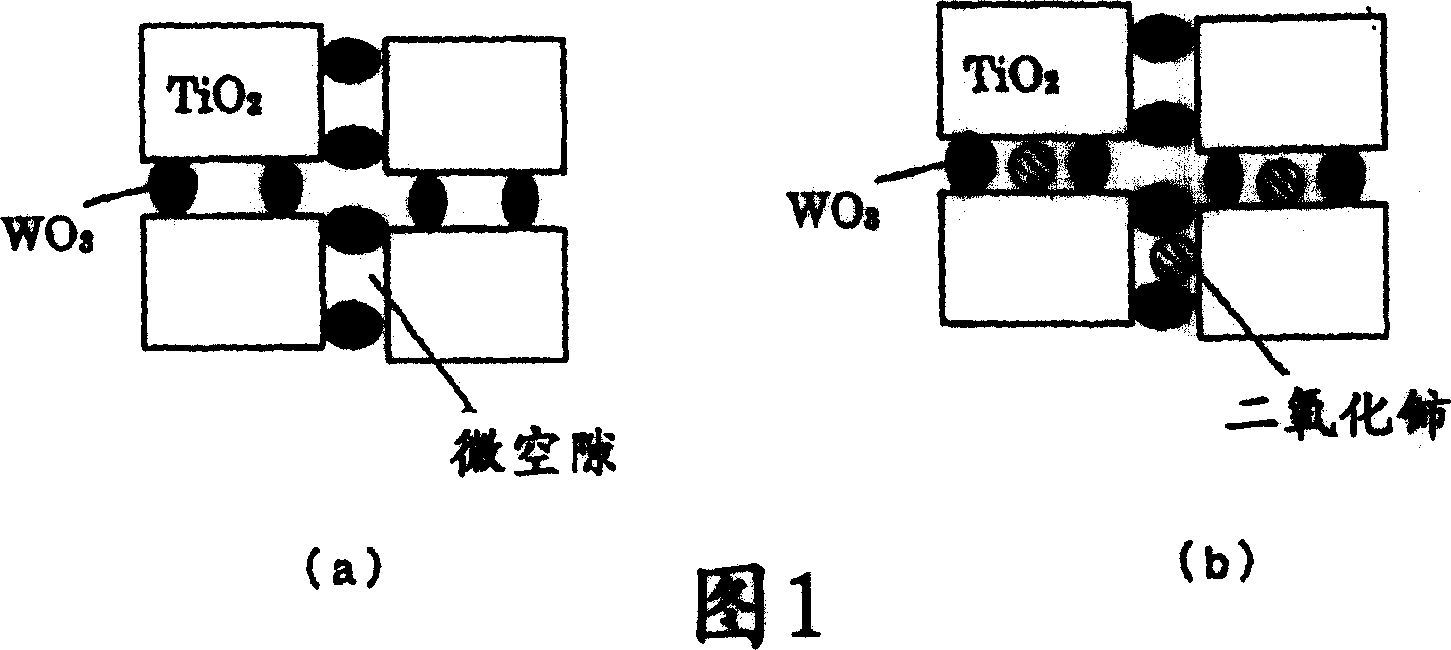
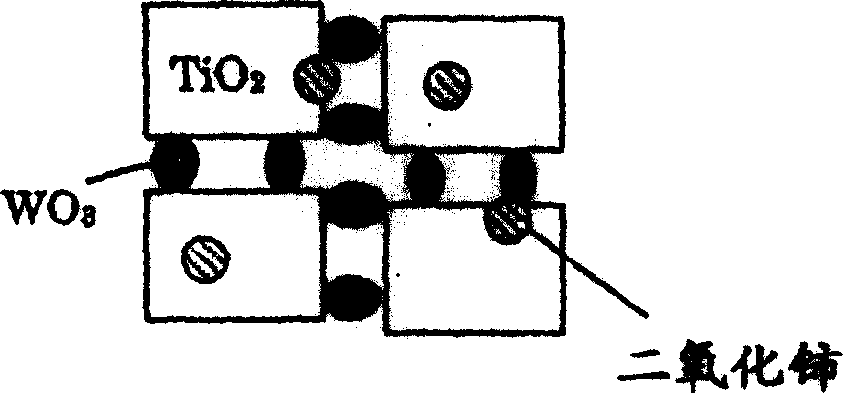
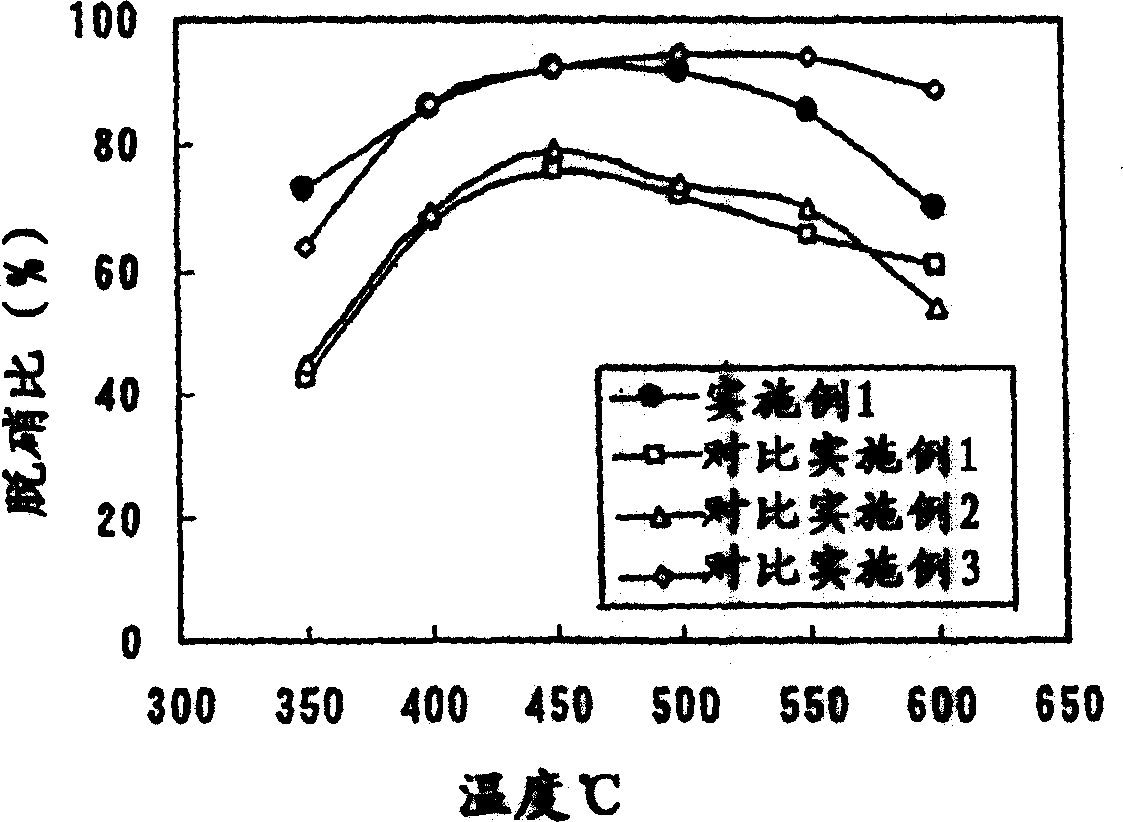
Catalyst for removing nitrogen oxides, method for production thereof and method for removing nitrogen oxides
A technology of nitrogen oxides and catalysts, which is applied in the field of catalysts and can solve problems such as catalyst degradation and high catalyst manufacturing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] 80 g of titanium dioxide dried at low temperature (produced by Millennium Co., Ltd., trade name: G5, surface area 275m 2 / g), 52.7g ammonium metatungstate aqueous solution ((NH 4 ) 6 ·H 2 W 12 o 40 ·xH 2 O, the concentration in the solution is expressed as WO 3 Calculated as 50wt%), 26.2g CeO 2 Sol (produced by Taki Chemical Co., Ltd., trade name: Needrahl, CeO 2 Content: 15wt%), 4g oxalic acid, 50g silica sol (produced by Nissan Chemical Ind., trade name: OS sol, concentration: 20wt%) and 50g water mix, prepare TiO 2 Catalyst slurry with a concentration of 30 wt%.
[0046] A corrugated honeycomb made of aluminosilicate type inorganic fibers (produced by Nichias Co., Ltd., trade name: No. 3722) was cut into 5 cm squares, and immersed in the catalyst slurry thus obtained so that the catalyst slurry It was supported between inorganic fibers and on the surface of the fibers, dried at 150° C., and then calcined at 600° C. for 2 hours to prepare a catalyst.
[0047...
Embodiment 2 and 3
[0049] Catalyst is prepared in the same way as in Example 1, except that ammonium metatungstate aqueous solution and CeO 2 The addition amount of sol changes to 83.8g and 27.6g (embodiment 2) respectively, or 24.9g and 24.8g (embodiment 3), but in two embodiments by adjusting the addition amount of water to make the TiO of catalyst slurry 2 The concentration was maintained at about 30 wt%.
[0050] The chemical compositions of the catalysts thus obtained are respectively Ti / W / Ce (83 / 15 / 2 (Example 2) and 93 / 5 / 2 (Example 3)), in atomic percent.
Embodiment 4 to 6
[0052] Catalyst is prepared in the same way as in Example 1, except that ammonium metatungstate aqueous solution and CeO 2 The addition amount of sol changes to 52.1g and 12.9g (embodiment 4) respectively, 53.3g and 39.7g (embodiment 5), or 54.9g and 67.8g (embodiment 6), but in each embodiment by adjusting water The amount of addition makes the TiO of the catalyst slurry 2 The concentration was maintained at about 30 wt%.
[0053] The chemical compositions of the catalysts thus obtained are respectively Ti / W / Ce (89 / 10 / 1 (Example 4), 87 / 10 / 3 (Example 5), and 85 / 10 / 5 (Example 6)), In atomic percent.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com