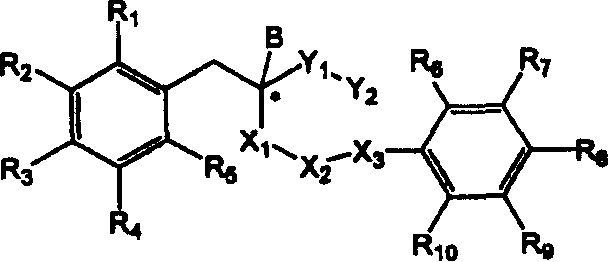
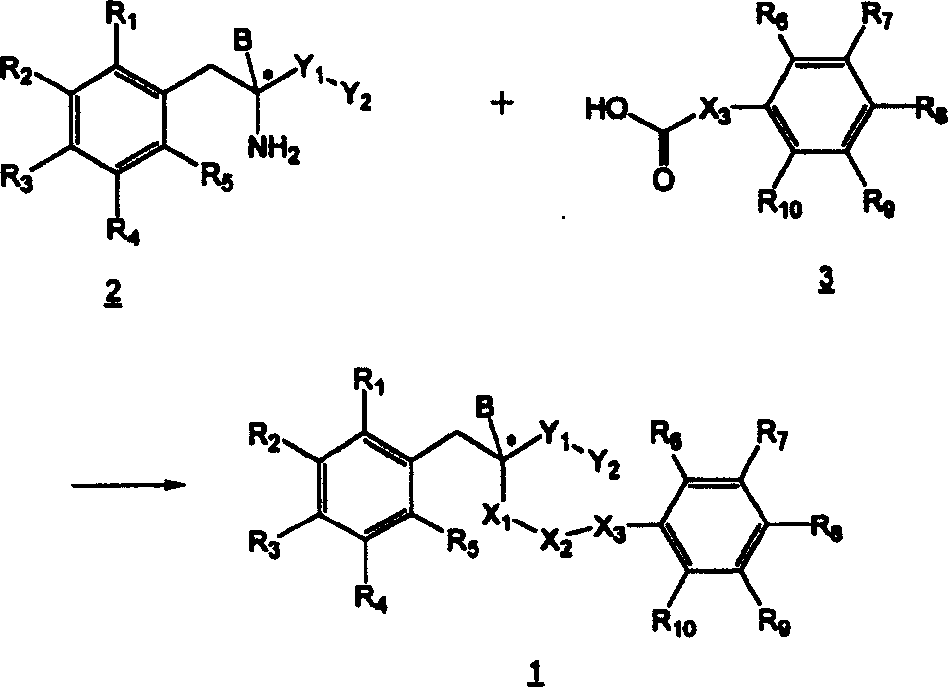
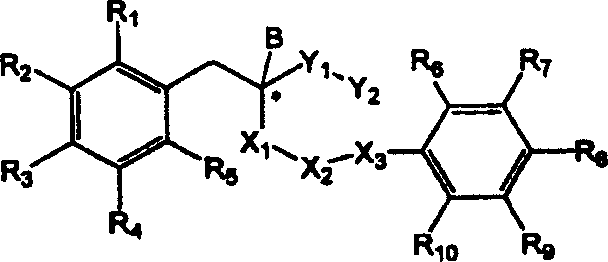
Derivatives of hydroxyphenyl, a method for preparing thereof and their pharmaceutical composition
A derivative and composition technology, applied in the field of hydroxyphenyl derivatives, can solve problems such as inability to recognize phosphotyrosine, loss of activated T cells, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0125] Preparation of 3-(3,4-dihydroxy-phenyl)-(R)-2-[3-trans-(3,4-dihydroxy-phenyl)-acrylamide]-propane methyl ester
[0126] (Step 1) Preparation of 3,4-dihydroxyphenyl-D-alanine methyl ester
[0127] Dissolve 2.0 g (10.14 mmol, 1 equivalent) of D-3,4-dihydroxyphenylalanine (D-DOPA) in 40 ml of methanol, and add thionyl chloride dropwise to the solution at 0° C. (7.4 ml, 101.4 mmole, 10 equivalents). The reaction mixture was stirred under nitrogen atmosphere for 18 hours and distilled under vacuum to remove excess methanol and thionyl chloride. The residue was recrystallized in methanol and ethyl acetate to provide DOPA methyl ester. The yield was 93%.
[0128] TLC (chloroform: acetone: methanol: water = 8: 8: 3: 1); Rf = 0.49
[0129] (Step 2) Preparation of 3-(3,4-dihydroxyphenyl)-(R)-2-[3-trans-(3,4-dihydroxy-phenyl)-acrylamido]-propionic acid methyl ester
[0130] In 10 ml of N,N-dimethylformamide, 2.0 g (8.07 mmole, 1 equivalent) of DOPA methyl ester obtained in...
Embodiment 2
[0134] Preparation of 3-(3,4-dihydroxy-phenyl)-(R)-2-[3-trans-(3,4-dihydroxy-phenyl)-acrylamide]-propane acid
[0135] 70 mg of 3-(3,4-dihydroxy-phenyl)-(R)-2-[3-trans-(3,4-dihydroxy-phenyl)-acrylamino]- Methyl propionate was dissolved in 50 ml of a mixed solvent containing acetone and water (4:25, v / v), and then 8 ml of HCl solution was added thereto. The reaction mixture was refluxed in an oil bath for 1 day, concentrated to remove acetone, and then ethyl acetate was added to give the title compound. The yield was 54%.
[0136] TLC (n-hexane:ethyl acetate:methanol=4:5:1) product Rf=0.28.
[0137] M / z 360.1(M+H)
[0138] 1 H NMR (DMSO-d 6 )δ8.62, 8.67, 9.07, 9.31 (4H, br, -OH), 8.75 (1H, d, J = 8.0Hz, NH), 7.20 (1H, d, J = 15.7Hz, CH), 6.93 (1H , d, J = 1.8Hz, aromatic), 6.74 (1H, d, J = 8.1Hz, aromatic), 6.62 (1H, d, J = 1.8Hz, aromatic), 6.61 (1H, J = 7.8Hz, aromatic), 6.57 (1H, d, J = 7.9, 1.8Hz, aromatic), 6.41 (1H, d, J = 15.7Hz, CH), 4.48 (1H, m, CH), 2.90 (1H...
Embodiment 3
[0139] Preparation of 3-(3,4-dihydroxy-phenyl)-(R)-2-[3-trans-(3,4-dihydroxy-phenyl)-acrylamido]-propane ethyl acetate
[0140] Except that ethanol is used as the reaction solvent instead of methanol in Step 1 of Example 1 to obtain D-DOPA ethyl ester, and D-DOPA ethyl ester is used as the starting material, the reaction is carried out in the same manner as described in Example 1, The title compound was obtained.
[0141] M / z 388.8(M+H)
[0142] 1 H NMR (DMSO-d6) δ8.62, 8.67, 9.07, 9.31 (4H, br, -OH), 8.75 (1H, d, J = 8.0Hz, NH), 7.20 (1H, d, J = 15.7Hz, CH), 6.93 (1H, d, J = 1.8Hz, aromatic), 6.74 (1H, d, J = 8.1Hz, aromatic), 6.62 (1H, d, J = 1.8Hz, aromatic) , 6.61 (1H, J=7.8Hz, aromatic), 6.57 (1H, d, J=7.9, 1.8Hz, aromatic), 6.41 (1H, d, J=15.7Hz, CH), 4.48 (1H , m, CH), 2.90 (1H, dd, J=13.7, 4.9Hz, CH 2 ), 2.72 (1H, dd, J=13.7, 4.9Hz, CH 2 )4.12 (2H, q, J = 10.1, CH 2 ) 1.30 (3H, t, J=10.1, CH 3 )
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com