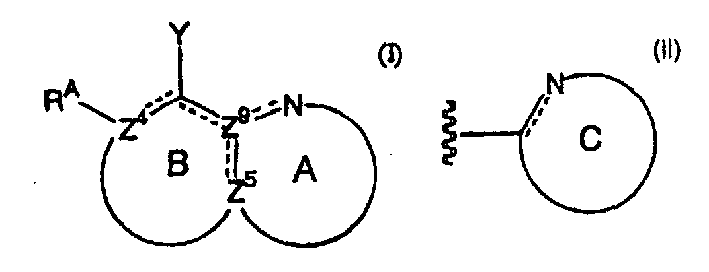
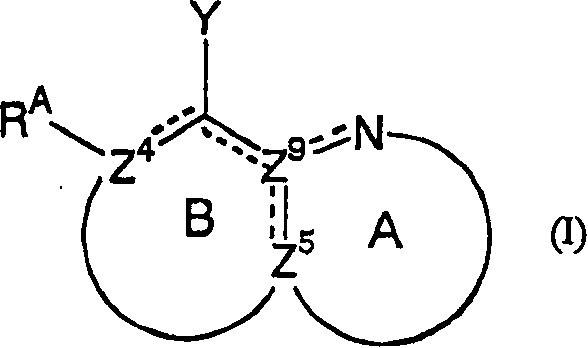
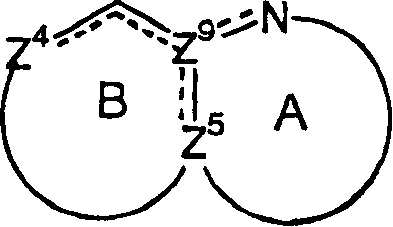
Nitrogen-containing heteroaryl compounds having HIV integrase inhibitory activity
A compound, heteroaryl technology, applied in the field of new compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0470] Compound I-1
[0471]
[0472] step 1
[0473] At room temperature, 4-acetylamino-5-chloro-2-methoxy-3-nitrobenzoic acid 1 (20.6 g, 67.9 mmol) in methanol (200 ml) was added 5N hydrochloric acid (60 ml), and the mixture was heated under reflux for 14 hours. The reaction solution was neutralized with 2N sodium hydroxide solution under ice-cooling, and then water (100 ml) was added, followed by stirring for 30 minutes. Precipitated crystals were collected and washed with water (100 ml) to obtain Compound 2 (11.9 g) as wet yellow crystals.
[0474] step 2
[0475] The suspension of compound 2 (11.9g), ammonium chloride (1.22g, 22.8mmol) and iron (10.2g, 183mmol) obtained in step 1 in ethanol (450ml)-water (90ml) was heated to reflux for 3 hours. The reaction mixture was filtered through celite, and the remaining residue was washed with chloroform-methanol (1:1 v / v, 500 ml). The filtrate was concentrated under reduced pressure, then water (100 ml) was added to the r...
Embodiment 2
[0521] Compound I-6
[0522]
[0523] step 1
[0524] Under ice cooling, sodium hydride (60%, 0.42g, 10.5mmol) was added to a solution of compound 1 (3.03g, 10.0mmol) in DMF (40ml) over 5 minutes, and the mixture was stirred at room temperature for 1 hour , and then added methyl iodide (0.685ml, 11.0mmol) under ice-cooling, and stirred at room temperature for 3 hours. The reaction mixture was adjusted to pH 3 with 10% hydrochloric acid under ice-cooling. Water (50ml) was added and extracted with ethyl acetate. The organic layer was washed with water (50ml) and brine (50ml), dried over sodium sulfate. The solvent was evaporated under reduced pressure to afford crude compound 6 as a dark brown oil.
[0525] step 2
[0526] Sodium methoxide (28% methanol solution, 3 ml) was added to a methanol (3 ml) solution of the crude compound 6 obtained in step 1, and the mixture was heated to reflux for 2 hours. Under ice-cooling, the reaction mixture was poured into 1N hydrochlori...
Embodiment 3
[0558] Compound I-10
[0559]
[0560] Steps 1-4
[0561] Compound I-10 was synthesized in the same manner as in Step 1 of Example 2 and Steps 2, 4, and 5 of Example 1.
[0562] Melting point: 168-170°C Recrystallization solvent: methanol-isopropanol
[0563] Elemental Analysis: C 17 h 14 ClFN 2 o 3
[0564] Calculated (%): C, 58.55; H, 4.05; N, 8.03; Cl, 10.17; F, 5.45.
[0565] Found value (%): C, 58.47; H, 3.97; N, 8.08; Cl, 9.90; F, 5.19.
[0566] NMR (DMSO-d 6 )δ: 2.49 (3H, s), 3.90 (3H, s), 5.74 (2H, s), 7.05 (2H, m), 7.18 (2H, m), 7.54 (1H, s), 11.10 (1H, brs ).
[0567] IR (KBr): 3417, 1699cm -1 .
[0568] Compound I-11 was synthesized in the same manner as in Example 3.
[0569]
[0570] Compound I-11
[0571] Melting point: 185-187°C Recrystallization solvent: ethyl acetate-hexane
[0572] Elemental Analysis: C 18 h 16 ClFN 2 o 3
[0573] Calculated (%): C, 59.59; H, 4.45; N, 7.72; Cl, 9.77; F, 5.24.
[0574] Found value (%): C, 59.59...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com