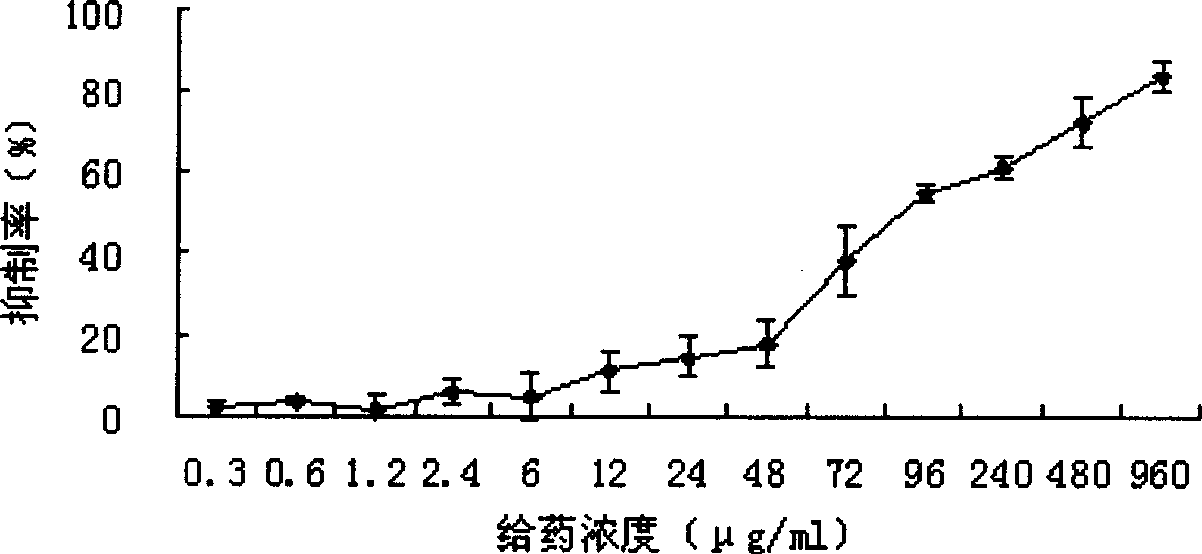
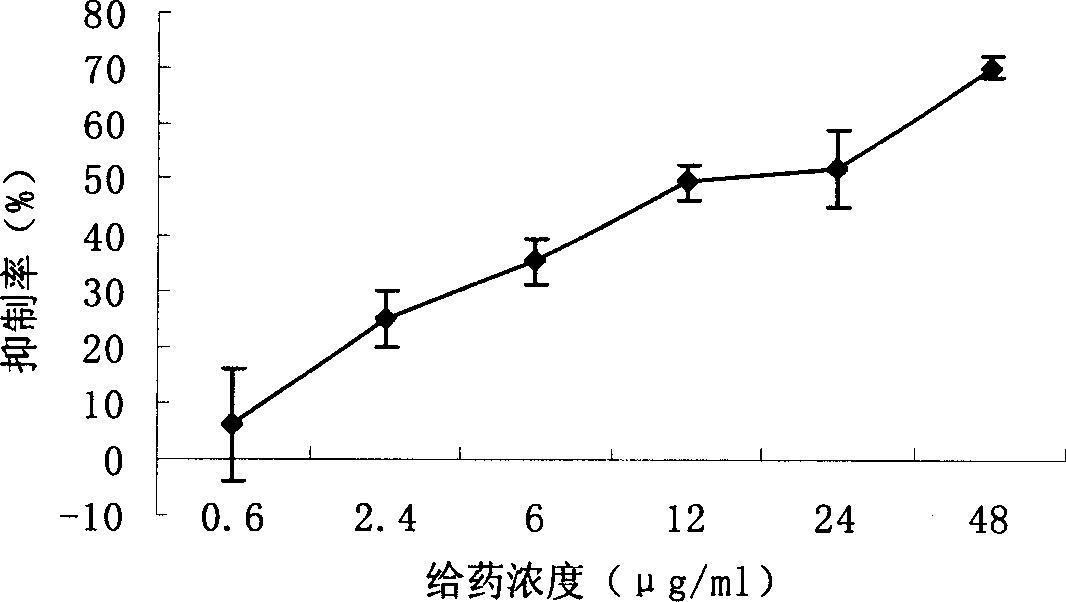
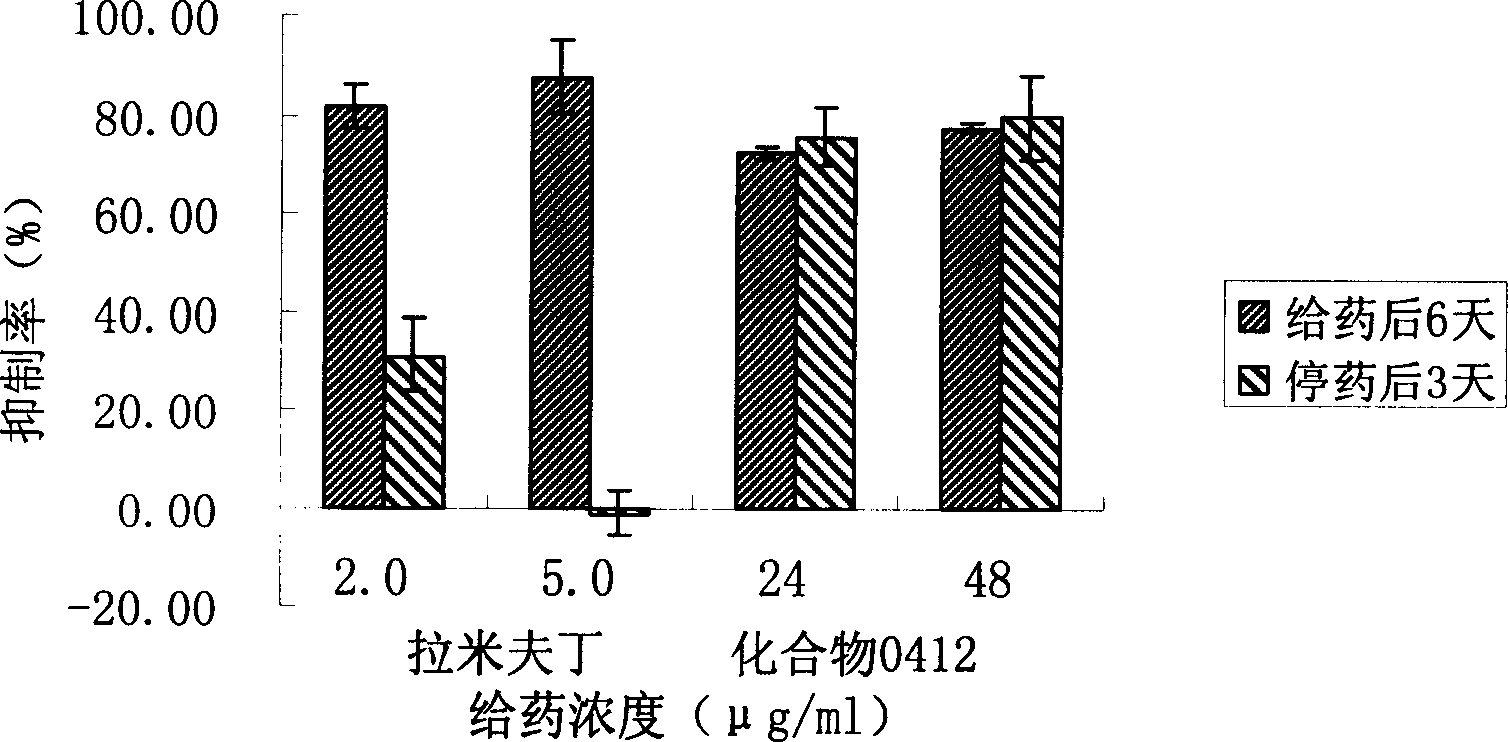
Novel use of 3, 4-dihydroxy-benzene formaldehyde and its derivatives in treating hypatitis B and virus infectious diseases
A technology of hydroxybenzoyl and derivatives, applied in 3, can solve problems such as no discovery, and achieve the effect of important social and economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] [Example 1] Preparation of substituted derivatives of compound 0412 (taking 3,4-dibenzoyloxybenzaldehyde as an example):
[0036] In an ice-salt bath, use dichloromethane as a solvent, add compound 0412 and triethylamine to dissolve, then add benzoyl chloride dropwise with stirring, keep the temperature below 5°C, and continue stirring for several hours after the drop; water, dilute hydrochloric acid, Wash with water, sodium bicarbonate solution and water; dry overnight with anhydrous sodium sulfate; concentrate under reduced pressure to obtain the crude product; recrystallize from ethanol water; dry to obtain the product. Other C1-6 acyl, caffeoyl, chlorogenic acyl and other compounds are synthesized with the above method.
Embodiment 2
[0037] [Example 2] Preparation of ester derivatives of compound 0412 (taking 3,4-dibenzoyloxyethyl benzoate as an example)
[0038] 3,4-Dibenzoyloxybenzoic acid and absolute ethanol are heated under acidic conditions, recrystallized from ethanol water, and dried to obtain the product. Other ester-forming reactions can be prepared by this method, and the introduction of groups at other positions can be seen in the preparation of substituted derivatives of compound 0412.
Embodiment 3
[0039] [Example 3] Preparation of acetal derivatives of compound 0412 (taking 3,4-dibenzoyloxybenzaldehyde ethylene acetal as an example)
[0040] Dissolve 3,4-dibenzoyloxybenzaldehyde in anhydrous toluene, add ethylene glycol and a catalyst amount of p-toluenesulfonic acid, and reflux for several hours under the Dean-Stark device; the solvent is evaporated by rotary evaporation; ethanol water Recrystallization; drying products. The synthesis method of other acetal groups is similar; for the introduction of groups at other positions, see the preparation of substituted derivatives of compound 0412.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com