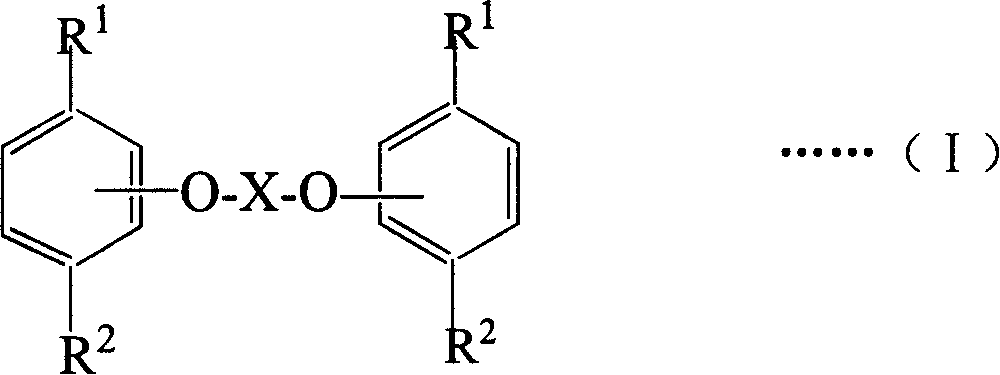
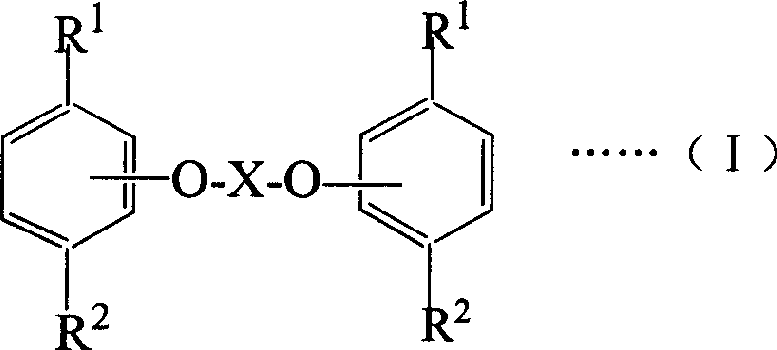
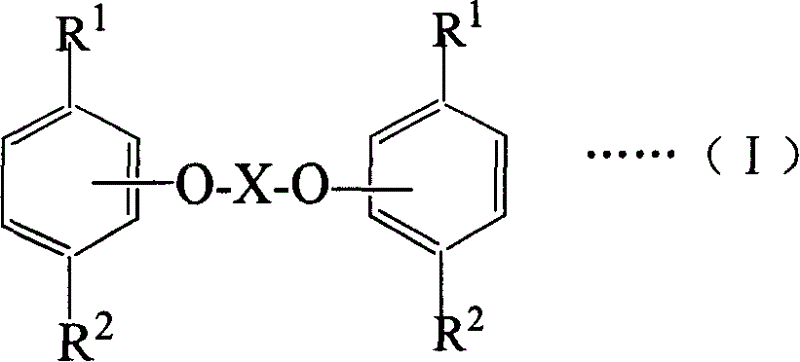Ester compound of thymol and/or carvacrol, preparing method and its medicinal active composition
A technology of ester compounds and pharmaceutical activity, which is applied in the field of preparation methods and pharmaceutically active compositions thereof, and in the field of ester compounds of thymol and/or carvacrol, which can solve the problem of affecting the pharmacokinetic process, slow absorption, affecting Efficacy and other issues, to achieve the effect of enhancing the antibacterial and anticoccidial effects and effects, improving distribution and metabolism, and increasing bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Synthesis of Thymol
[0058] Suspend 400g of anhydrous aluminum trichloride in 900g of 1,2-dichloroethane, cool to -9~-10°C, add a mixture of 216g of m-cresol-1,2-dichloroethane and 200g of it dropwise In the anhydrous aluminum chloride suspension, keep the temperature at -10°C, add 174g of 2-chloropropane dropwise and keep the temperature at -5~-10°C to react for 8~10 hours, and detect the end point of the reaction by thin layer chromatography. After the clarified reaction solution was poured into crushed ice for ice-thawing, the ice-thawed solution was poured into a separatory funnel, and the lower layer was separated. The aqueous layer was extracted three times with 1,2-dichloroethane, 50 ml each time, and the organic layers were combined. The organic layer was washed with water until neutral, the washing liquid was extracted once with a small amount of 1,2-dichloroethane, the organic layers were combined, washed with saturated sodium chloride solution, and dried wit...
Embodiment 2
[0065] Synthesis of Carvacrol
[0066] ①Limonene nitrosyl chloride preparation
[0067] Dissolve 40.8g of limonene (L-limonene) in 40ml of isopropanol, and cool the solution to below 10°C. Dissolve 120ml of concentrated hydrochloric acid in 80ml of isopropanol, and 20.7g of saturated aqueous solution of sodium nitrite, and simultaneously add limonene-isopropanol solution through the dropping funnel to control the reaction temperature below 10°C, stir for 15 minutes, and keep warm for one Filter for 1 hour, wash the solid with cold butanol, yield 50 g, and recrystallize with chloroform-methanol (1:3), melting point: 103°C.
[0068] ② Preparation of carceloxime
[0069] 40g of limonene nitrosyl chloride (crude product), 20ml of dimethylformamide (DMF) and 125ml of isopropanol were placed in a four-necked flask and refluxed for 30 minutes. After filtration, the solid was washed three times with 50 ml of ice water and once with 15 ml of cold isopropanol, and dried to obtain 27 ...
Embodiment 3
[0079] Preparation of Di-Thymolyl Thiocarbonate
[0080] Put 31.54g of thymol and 300ml of chloroform in a four-necked bottle, under stirring, the temperature is controlled at -6~-8°C, add thiophosgene dropwise, and then add dropwise a solution of 6g of sodium hydroxide, 1.2g of sodium sulfide in water and 81ml of water , the temperature was controlled below -5~-7°C, after dropping, it was raised to room temperature, and continued to stir for 2 to 4 hours. The end point of the reaction was controlled by thin-layer chromatography, and the organic layer was separated, and the aqueous layer was extracted twice with chloroform. The aqueous layer and the chloroform layer were combined, dried with anhydrous sodium sulfate, the chloroform was recovered, and the residual liquid was subjected to column separation. Using 1:10 silica gel and petroleum ether as a developer, collect petroleum ether solution, concentrate under reduced pressure to obtain a brown-red crude product, then disti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com