Notoginseng total saponin slow release preparation
A technology of panax notoginseng total saponins and slow-release preparations, which is applied to medical preparations containing active ingredients, cardiovascular system diseases, organic active ingredients, etc., and can solve the problem of low bioavailability of PNS oral preparations, affecting the labor force and economic growth , allergic reactions and other problems, to avoid drug side effects, improve bioavailability, and enhance the effect of efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
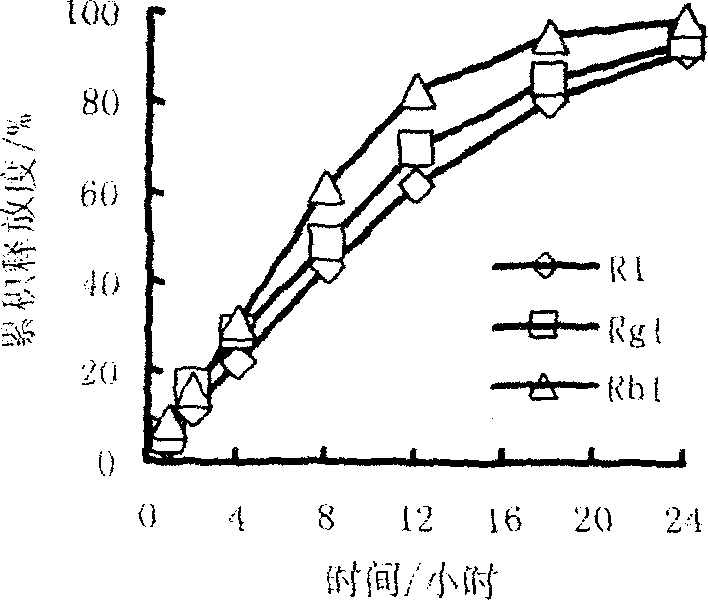
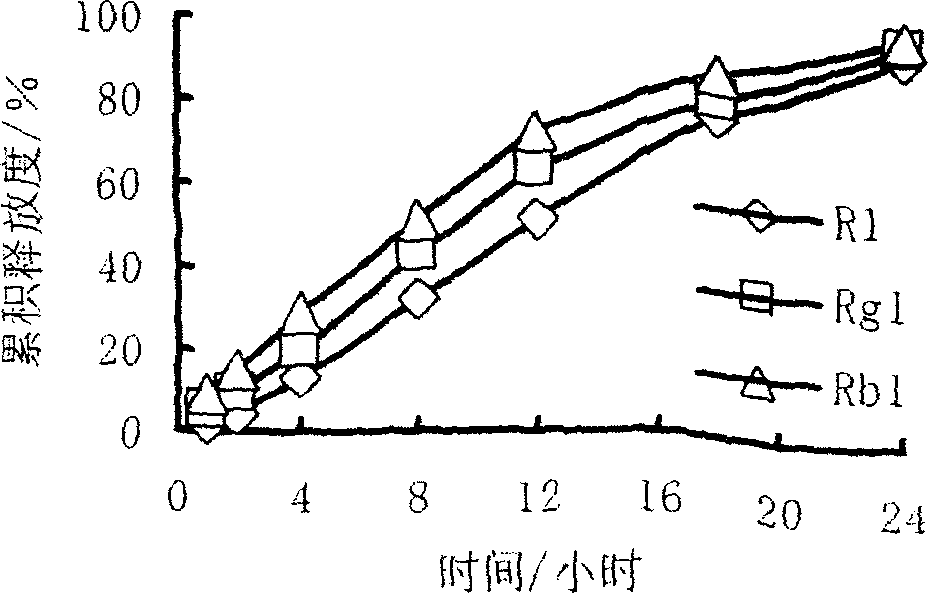
[0028] According to the calculation of the preparation of 1000 tablets, take 70g of Panax notoginseng saponins, 30g of hypromellose, 0.2g of polyethylene glycol 4000, an appropriate amount of 95% medicinal alcohol, and 2g of magnesium stearate to make 1000 tablets in total. Specifications: Each tablet contains 70mg of Panax notoginseng saponins, and the theoretical tablet weight is about 102mg. The raw materials were passed through a 100-mesh sieve respectively, and the total saponins of Panax notoginseng and hypromellose were weighed according to the prescription and mixed evenly; 95% medicinal alcohol was used as a wetting agent to make soft materials, 24-mesh sieve was granulated, and vacuum dried at 60 ° C for 3 hours , 24-mesh sieve and granulated, add magnesium stearate and mix well, press with a 7mm punch to obtain the slow-release tablet of Panax notoginseng total saponins of the present invention. The 24-hour release rate was 75%.
Embodiment 2
[0030] According to the calculation of the preparation of 1000 tablets, take 60g of Panax notoginseng saponins, 40g of hypromellose, 0.4g of soluble starch, appropriate amount of 95% medicinal alcohol, and 2g of magnesium stearate to make a total of 1000 tablets. Tablet specifications: each The tablet contains 60 mg of Panax notoginseng saponins, and the theoretical tablet weight is about 102 mg. The raw materials were passed through a 100-mesh sieve respectively, and the total saponins of Panax notoginseng and hypromellose were weighed according to the prescription and mixed evenly; 95% medicinal alcohol was used as a wetting agent to make soft materials, 24-mesh sieve was granulated, and vacuum dried at 60 ° C for 3 hours , 24-mesh sieve and granulated, add magnesium stearate and mix well, press with a 7mm punch to obtain the slow-release tablet of Panax notoginseng total saponins of the present invention. The 24-hour release rate was 85%.
Embodiment 3
[0032] According to the calculation of the preparation of 1000 tablets, take 90 g of Panax notoginseng saponins, 5 g of hypromellose, 5 g of ethyl cellulose, 0.8 g of polyethylene glycol 4000, an appropriate amount of 95% medicinal alcohol, and 2 g of magnesium stearate. It is made into 1000 tablets, tablet specifications: each tablet contains 90 mg of Panax notoginseng saponins, and the theoretical tablet weight is about 102 mg. The raw materials were passed through a 100-mesh sieve respectively, and the total saponins of Panax notoginseng, hypromellose, and ethyl cellulose were weighed according to the prescription and mixed evenly; 95% medicinal alcohol was used as a wetting agent to make soft materials, and 24-mesh sieve was used to granulate at 60°C. Under vacuum drying for 3 hours, 24-mesh sieve for granulation, adding magnesium stearate and mixing, and pressing with a 7mm punch to obtain the Panax notoginseng total saponins sustained-release tablet of the present inventi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com