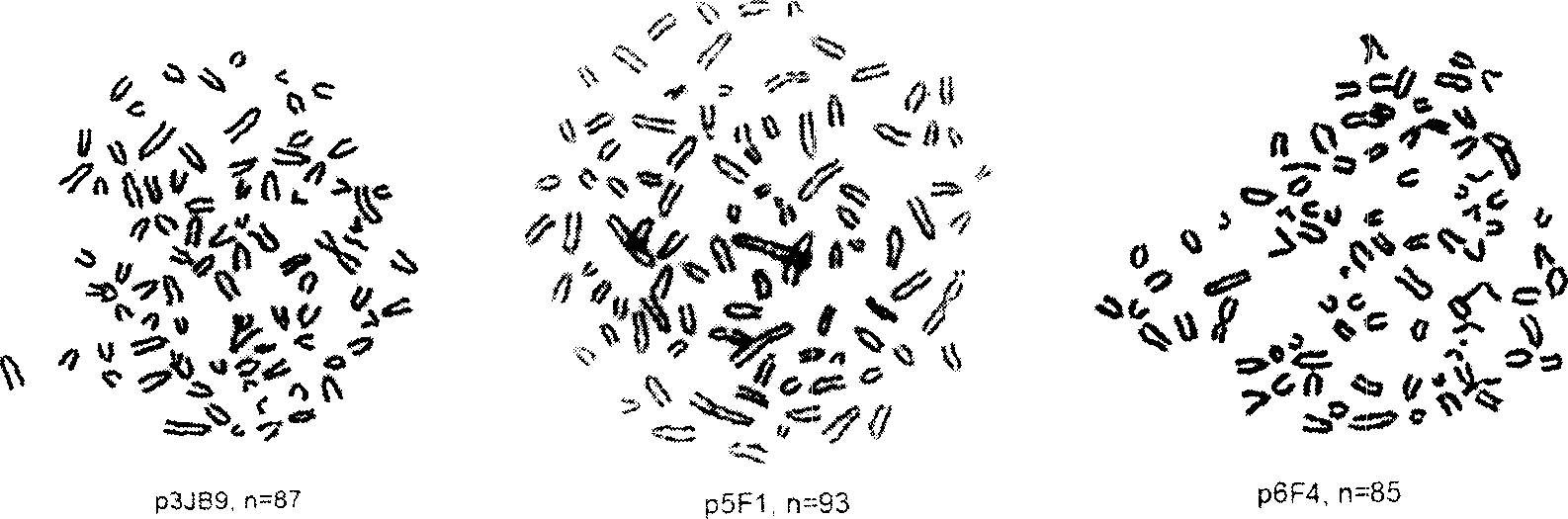Monoclonal antibody of anti human immune deficiency virus I type 24 protein and application thereof
A technology of immunodeficiency virus and monoclonal antibody, which is applied in the direction of antiviral immunoglobulin, measuring devices, instruments, etc., can solve the problems of no public reports and increase of non-specific reactions, and achieve high sensitivity, strong specificity, and preparation The effect of simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1, the preparation of monoclonal antibody
[0037] (1) Immunized mice
[0038] The immunogen used to prepare the monoclonal antibody is the purified subtype B HIV-1p24 genetically engineered recombinant protein. The full-length protein of subtype B HIV-1p24 was expressed in E.coli expression system expressing HIV-1B subtype p24 protein in large quantities, and the GST-p24 fusion protein was expressed in large quantities. After the bacteria were lysed with lysozyme, the Parmacia Sepharose-4B GST affinity chromatography column was used. GST fusion protein was adsorbed, digested with PreScission protease at 4°C overnight, and eluted with elution buffer to obtain electrophoretic pure HIV-1B subtype recombinant p24 protein.
[0039] BALB / c mice were immunized with subtype B HIV-1p24 recombinant protein, and the specific immunization scheme was as follows:
[0040] HIV-1p24 antigen plus complete Freund's adjuvant for initial immunization, 50ug / body
[0041] ...
Embodiment 2
[0060] Embodiment 2, identification of monoclonal antibody
[0061] fusion cell chromosome count
[0062] The confluent cells cultured into a single layer were cultured with colchicine (0.1 μg / ml) for 36-48 hours, treated with 0.075 M KCI for hypotonicity, fixed with glacial acetic acid and methanol, prepared into slices and stained with Giemsa. The chromosomes of 20 effective cells were counted in each of the three hybridomas. The average number of chromosomes in hybridoma p3JB9 was 89.7±5.5, the average number of chromosomes in p5F1 was 91.0±1.5 and the average number in p6F4 was 83.2±4.6.
[0063] Karyotype analysis of three hybridoma cells figure 1 shown.
[0064] Identification of monoclonal antibody Ig class / subclass
[0065] With plating solution (Na 2 CO 3 1.59g / l, NaHCO 3 2.93g / l, pH 9.6) Dilute the p24 antigen to 1μg / ml, 100μl / well, overnight at 4°C; wash the plate with washing solution, seal the plate with blocking solution (5% milk powder, PBS, pH 7.4), and...
Embodiment 3
[0079] Example 3, mass production of monoclonal antibodies
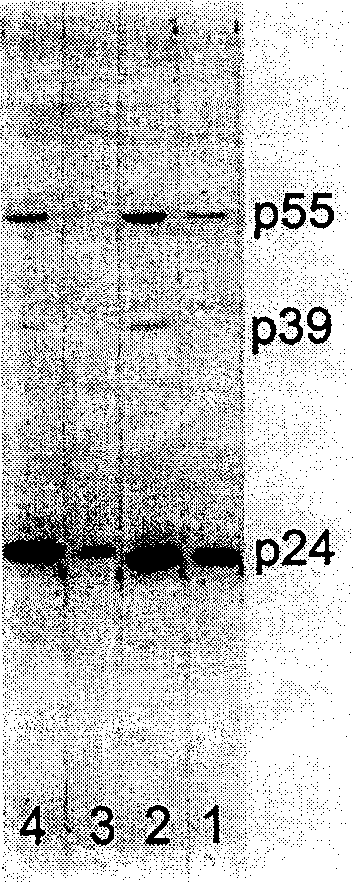
[0080] First intraperitoneally inject 0.5ml Pristane (pristane) to BALB / c mice, and intraperitoneally inject O.5×10 6 Ascites can be produced 7-10 days after the inoculation of the cells, the mice were sacrificed, the ascites was extracted with a syringe, the supernatant was obtained by centrifugation, and stored at -20°C. The content of monoclonal antibody in ascites can reach 5-20mg / ml.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com