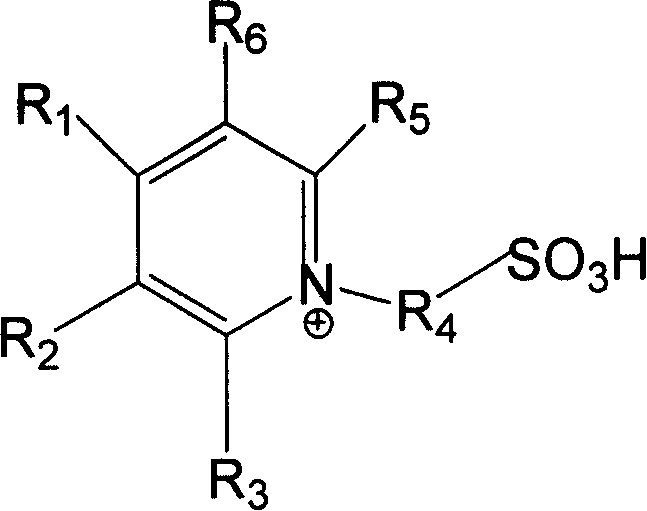
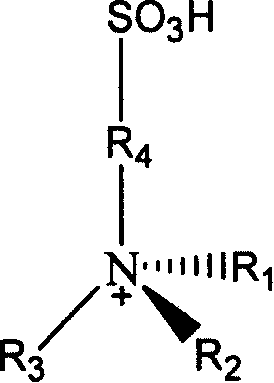
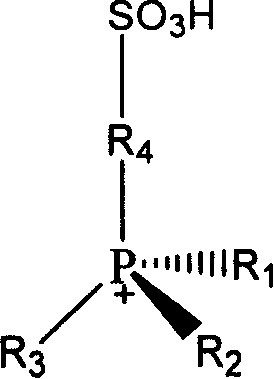
Method for synthesizing D,L-alpha-tocopherol catalyzed by ion-liquid
An ionic liquid, tocopherol technology, applied in chemical instruments and methods, physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, etc., can solve problems such as toxicity, catalyst corrosion, poor activity, etc. Achieve the effect of high conversion rate, high purity and easy separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Implementation Example 1: Take 0.051 mole of N-propanesulfonic acid pyridinium-boron tetrafluoroide ionic liquid (water content is 15%) and 0.033 mole of trimethylhydroquinone into the reactor and stir to mix evenly. Heat to 150°C, add 0.013 moles of isophytol dropwise to the above mixture under continuous stirring (the molar ratio of ionic liquid, trimethylhydroquinone and isophytol is about 5:3:1), dropwise The time is 1h, and the heating and stirring reaction is continued for 0.5h. After the reaction was completed, petroleum ether was extracted 5 times with 20ml each time, and the extract was distilled under reduced pressure at 50°C to evaporate the solvent to obtain the product D, L-α-tocopherol. The yield was 66.2%.
Embodiment 2
[0054] Implementation Example 2: Take 0.052 moles of N-propanesulfonic acid pyridinium-sulfuric acid ionic liquid (water content is 5.6%) and 0.0234 moles of trimethylhydroquinone into the reactor and stir to mix evenly. Heat to 115°C, add 0.021 moles of isophytol dropwise to the above mixture under continuous stirring (the molar ratio of the three materials of ionic liquid, trimethylhydroquinone and isophytol is about 2:1:1), dropwise The time is 60 minutes. Continue to heat and stir the reaction for 10h. After the reaction was completed, ethyl acetate was extracted three times with 20ml each time, and the extract was distilled under reduced pressure at 50°C to evaporate the solvent to obtain the product D, L-α-tocopherol. Yield 52.6%.
Embodiment 3
[0055] Implementation Example 3: Take 0.049 moles of N-propanesulfonic acid pyridine-p-toluenesulfonic acid ionic liquid (water content is about 6%) and 0.0244 moles of trimethylhydroquinone into the reactor and stir to mix evenly. Heat to 115°C, add 0.0221 moles of isophytol dropwise to the above mixture under continuous stirring (the molar ratio of ionic liquid, trimethylhydroquinone and isophytol is about 2:1:1), dropwise The time was 40min, and the heating and stirring reaction was continued for 2h. After the reaction was completed, ethyl acetate was extracted three times with 20ml each time, and the extract was distilled under reduced pressure at 50°C to evaporate the solvent to obtain the product D, L-α-tocopherol. Yield 9.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com