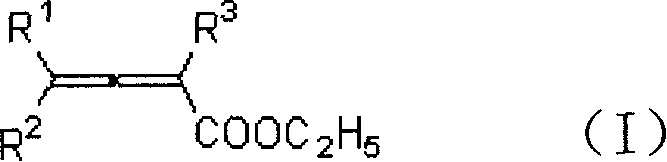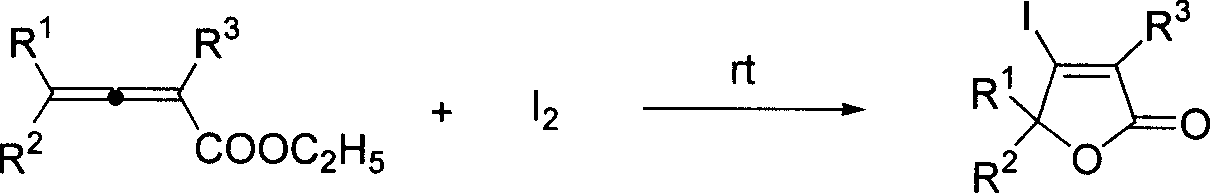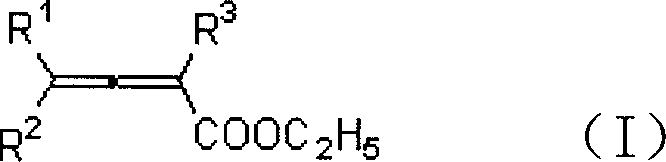Method for synthesizing beta-iodobutyl lactone
A technology of iodobutenoic acid lactone and allenoic acid ester is applied in the field of synthesizing β-iodobutenoic acid lactone, can solve the problems of low hydrolysis yield, isomerization and the like, and achieves low cost and high reaction efficiency. Short time, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add ethyl 2,3-heptadienoate (81.9mg, 0.53mmol), MeCN (4mL), water (0.27mL) into a 25mL egg-shaped flask, add iodine (254mg, 1.0mmol) under stirring, and continue to The reaction was stirred for 12 hours. Water (6 mL) was added to the reaction solution to quench, and saturated sodium thiosulfate eliminated excess iodine. Extract with ether, dry over anhydrous sodium sulfate, filter, concentrate, and perform flash column chromatography to obtain 94.9 mg of the product 3-iodo-γ-heptenolactone with a yield of 71%. The product was a white solid with a melting point of 57-58°C.
[0021] 1 H NMR (400MHz, CDCl 3 )δ6.45(s, 1H), 4.89-4.92(m, 1H), 1.67-1.98(m, 1H), 1.50-1.55(m, 1H), 1.35-1.42(m, 2H), 0.91(t, J=6Hz, 3H);
[0022] 13 C NMR (100MHz, CDCl 3 )δ171.1, 129.9, 125.5, 87.9, 34.6, 17.3, 13.7;
[0023] MS (70eV, EI) m / z (%): 253 (M + +1, 100);
[0024] IR(KBr)v(cm -1 ) 1738, 1582, 1296, 1168.
Embodiment 2
[0026] Add 2,3-heptadienoic acid ethyl ester (81.4mg, 0.52mmol), MeCN (4mL), water (0.27mL) in 25mL egg-shaped bottle, add iodine (381.8mg, 1.5mmol) under stirring, continue to The reaction was stirred at room temperature for 12 hours. The rest were the same as in Example 1, and 94.7 mg of the product 3-iodo-γ-heptenolactone was obtained with a yield of 71%. The product was a white solid with a melting point of 57-58°C.
Embodiment 3
[0028] Add 2,3-heptadienoic acid ethyl ester (81.3mg, 0.52mmol), MeCN (4mL), water (0.27mL) in 25mL egg-shaped bottle, add iodine (129.2mg, 0.5mmol) under stirring, continue to The reaction was stirred at room temperature for 12 hours. The rest were the same as in Example 1, and 88.2 mg of the product 3-iodo-γ-heptenolactone was obtained with a yield of 66%. The product was a white solid with a melting point of 57-58°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com