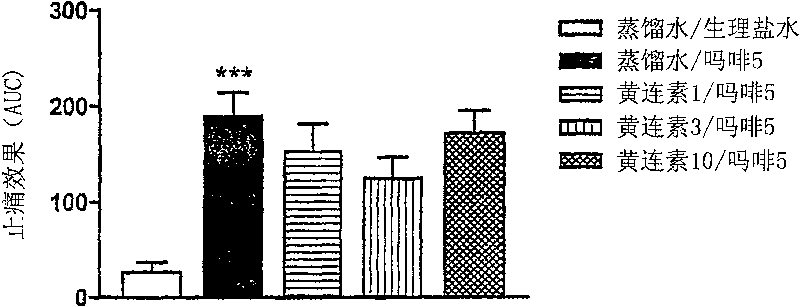Medicament component of berberine for the use of prevention and treatment of psycological dependence on and analgesic tolerance to morphine
A composition, tolerance technology, applied in the field of pharmaceutical compositions with harmful effects, can solve the problems of application restrictions, reduction of analgesic effect, dosage increase, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
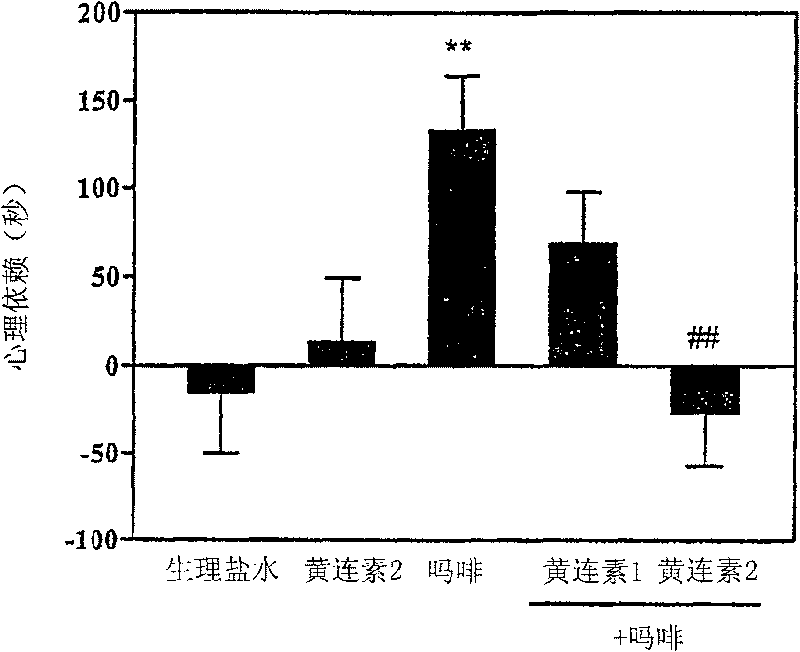
[0034] Example 1: Evaluation of the inhibitory effect of berberine on psychological dependence on morphine
[0035] The development of psychological dependence on morphine was detected by the conditioned place preference test.
[0036] The conditioned place preference test was performed in two equally sized boxes (15 x 15 x 15 cm), each with a front surface made of a transparent polypropylene sheet. The other three surfaces of each case are made of white or black polypropylene panels. The two boxes are connected by a gray passage (3×3×7.5 cm), and this passage can be blocked with a withdrawable gate.
[0037] To allow the mice to perceive the texture of the box floor, the white box had a rough floor and the black box had a smooth floor. Mice were cultured under a light intensity of 20 Lux.
[0038] Step 1 (preprocessing stage)
[0039] On day 1, the gate of the box was opened and the mice were allowed to freely explore both compartments for 5 min. On day 2, mice were hous...
Embodiment 2
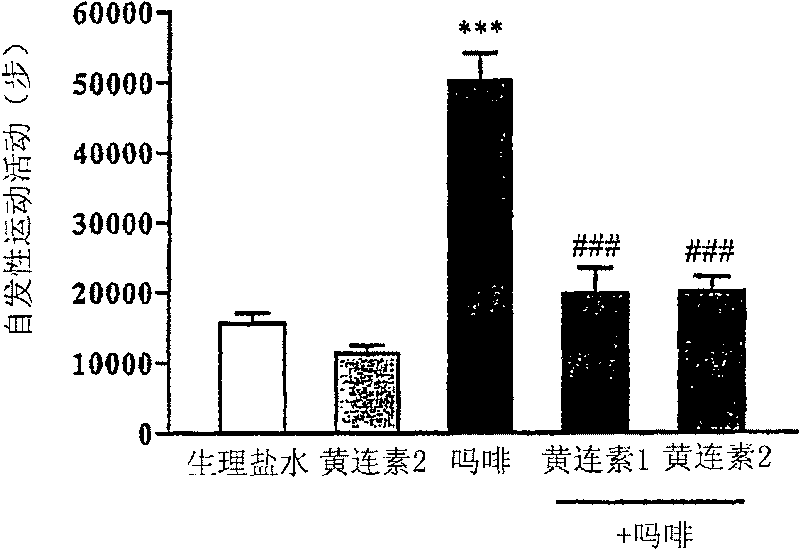
[0044] Example 2: Evaluation of the inhibitory effect of berberine on the increase in spontaneous locomotor activity induced by morphine
[0045] Spontaneous locomotor activity tests were performed in a plastic chamber (26 x 30 x 30 cm) using a video tracking system. First, 10 mg / kg of morphine was administered to mice once a day for 6 days. Immediately after the last dose, mice were placed in a plastic chamber, and spontaneous locomotor activity was recorded for 30 min and analyzed using a computer program. Berberine was orally administered to mice at 1 and 2 mg / kg 1 hour before morphine administration. The results are shown in figure 2 middle. Such as figure 2 As shown, when only morphine was administered, the locomotor activity of the mice increased significantly, with 50,242 forefoot steps. In contrast, when 1 and 2 mg / kg of berberine were pre-administered, the locomotor activity of the mice was 19,744 steps and 20,027 steps, respectively. In other words, compared ...
Embodiment 3
[0046] Example 3: Evaluation of the effect of berberine on the analgesic effect of morphine in a single dose
[0047] Male ICR mice, weighing 18-25 g, were acclimatized to the new environment by culturing in a temperature- and humidity-controlled vivarium for one week. 10 mice were used in each group. Morphine and berberine (berberine hemisulfate) were purchased from Keukdong Pharm Company (Inchon, Korea) and Sigma Company (USA), respectively, and dissolved in distilled water before use.
[0048] The effect of berberine on the analgesic effect of morphine was evaluated by applying heat stimulation to mice by hot plate test. For the hot plate test, each mouse was placed on a hot plate at 52°C and the latency until the mouse first showed signs of discomfort (hind paw flapping or jumping) was observed. To avoid tissue damage, a maximum heat exposure time (cut-off time) was artificially enforced, which was 30 seconds. If no behavioral response was shown for more than 30 seconds...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com