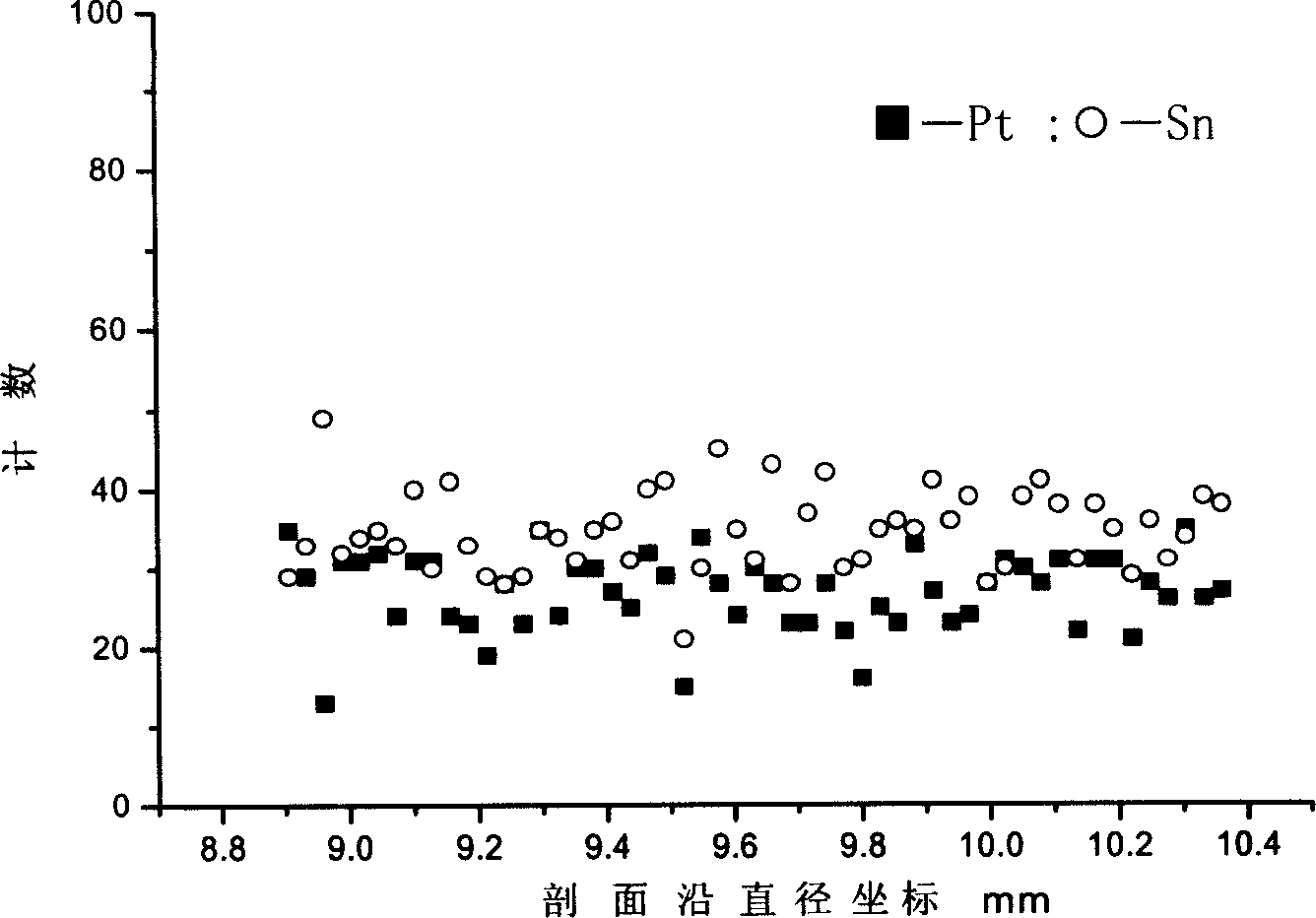
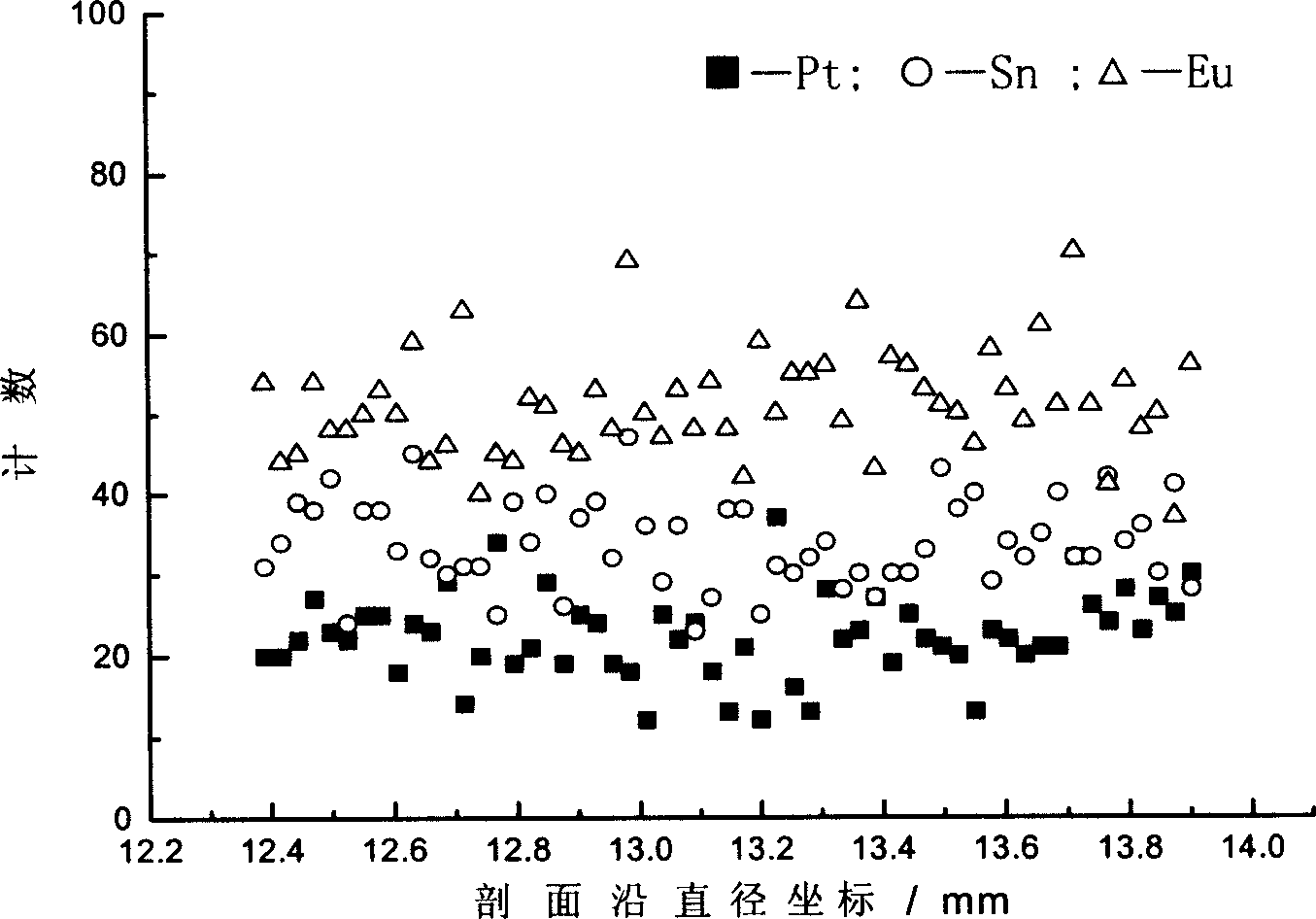
Process for preparing poly metal reforming catalyst
A reforming catalyst and catalyst technology, applied in the catalytic reforming of naphtha, etc., can solve the problems of rare earth metal loss, reduced catalytic effect, and low rare earth metal content, and achieve the effect of uniform distribution and preventing loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1~4
[0037] (1) Preparation of tin-containing alumina carrier
[0038] Prepare the carrier according to the method of example 1 in CN1150169A, get 100 grams of SB aluminum hydroxide powder (Germany, produced by Condea company) and an appropriate amount of deionized water, make the liquid / solid mass ratio 2.0, stir to make it slurry. Add the nitric acid that volume ratio is 1: 1, and its addition is 7.5 milliliters / 100 gram SB powders, adds the tin chloride of 30 gram urea and predetermined amount simultaneously, and the content of Sn is 0.30 mass % (calculated with dry basis alumina Standard, hereinafter the same), stirred for 1 hour, then added 30 grams of kerosene and 3 grams of fatty alcohol polyoxyethylene ether and stirred for 1 hour, then dropped the ball into the oily ammonia column for molding. The wet bulb is solidified in ammonia water for 1 hour, then filtered, rinsed with deionized water for 2 to 3 times, dried at 60°C for 6 hours, dried at 120°C for 10 hours, and calci...
example 5
[0049] Prepare the aluminum oxide carrier SC that contains tin and cerium by the method for example 1 (1), difference is when adding urea, replace tin chloride with tin chloride and cerium chloride, control the content of Sn to be 0.30 quality %, The content of cerium was 0.2% by mass. The carrier SC contains 0.3% by mass of Sn and 0.2% by mass of Ce.
[0050] Get 100 grams of carrier SC, impregnate with a solution containing 180 milliliters of diethylamine, HCl and chloroplatinic acid, the content of HCl in the impregnating solution is 2.5 mass %, and the platinum content is 0.30 mass % (both based on dry base alumina ), the ratio of the amount of diethylamine to HCl is 0.2, the soaking time is 24 hours, the mother liquor is separated from the solid with an acid-resistant filter funnel, the solid is dried at 120 ° C for 5 hours, and the solid is dried at 510 ° C in H 2 The O / HCl molecular ratio is 40: 1 water chlorine activation treatment in the air for 3 hours, the active c...
example 6
[0052] Catalyst PSR-8 was prepared according to the method of Example 5, except that the ratio of the amount of diethylamine to HCl in the impregnation solution was 0.4. The content of active components of PSR-8 is shown in Table 2. It can be seen from Table 2 that the rare earth Ce no longer dissolves during the impregnation process, and the platinum content in the catalyst decreases slightly.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com