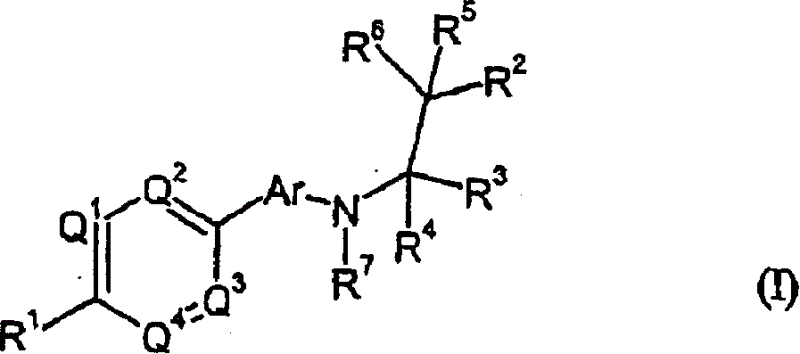
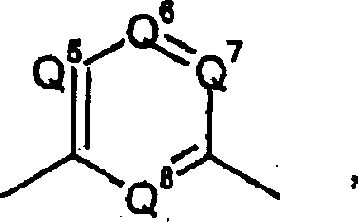
Phenyl or heteroaryl amino alkane derivatives as IP receptor antagonist
A technology for heteroarylaminoalkane and derivatives, which is applied in the field of phenyl or heteroarylaminoalkane derivatives, can solve the problems of undisclosed phenyl or heteroarylaminoalkane derivatives, and achieve excellent IP receptor antagonism Active, disease-relieving effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0476] N-(6-chloropyrimidin-4-yl)-D-phenylalanine methyl ester
[0477]
[0478] To a mixture of 4,6-dichloropyrimidine (57 g, 383 mmol), D-propylalanine methyl ester hydrochloride (75 g, 348 mmol) and 1,4-dioxane (440 mL) was added N , N-diisopropylethylamine (123ML, 730mmol), and the mixture was stirred overnight at 80°C. After cooling to room temperature, the mixture was concentrated under reduced pressure, and the residue was partitioned between ethyl acetate and water. The separated organic phase was washed with brine, dried over sodium sulfate, filtered and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography (hexane / ethyl acetate 3:1) to obtain N-(6-chloropyrimidin-4-yl)-D-phenylalanine methyl ester (99.3 g, 96 %), as a brown oil.
[0479] N-{6-[4-(Benzyloxy)phenyl]pyrimidin-4-yl}-D-phenylalanine methyl ester
[0480]
[0481] Under argon atmosphere, N-(6-chloropyrimidin-4-yl)-D-phenylalanine methyl ester (30....
Embodiment 1-2
[0493] N-{6-[4-(cyclopropylmethoxy)phenyl]pyrimidin-4-yl}-D-phenylalanine methyl ester
[0494]
[0495] Under argon atmosphere, N-(6-chloropyrimidin-4-yl)-D-phenylalanine methyl ester (1.27 g, 4.34 mmol), 4-(cyclopropylmethoxy)phenylboronic acid [ To a mixture of starting compound 1A] (1.0 g, 5.21 mmol) and benzene (8.7 mL) was added potassium carbonate (1.2 g, 8.68 mmol) followed by tetrakis(triphenylphosphine)palladium (0.25 g, 0.22 mmol). The mixture was stirred overnight at reflux. After cooling to room temperature, the mixture was filtered through a pad of celite, and the filtrate was partitioned between ethyl acetate and water. The separated organic phase was washed with brine, dried over sodium sulfate, filtered and concentrated under reduced pressure. The crude product was purified via silica gel column chromatography (hexane:ethyl acetate 8:2) to obtain N-{6-[4-(cyclopropylmethoxy)phenyl]pyrimidin-4-yl}-D- Phenylalanine methyl ester (1.05 g, 60%), as pale yello...
Embodiment 1-3
[0507] D-norleucine ethyl ester hydrochloride
[0508]
[0509]A solution of D-norleucine (1.50 g, 114 mmol) in ethanol (300 mL) was cooled to -70°C, and thionyl chloride (25.0 mL, 343 mmol) was added dropwise over 30 minutes. The mixture was heated at reflux overnight. After cooling to room temperature, the mixture was concentrated under reduced pressure to obtain D-norleucine ethyl ester hydrochloride (22.2 g, quantitative yield) as a colorless solid.
[0510] N-(6-chloropyrimidin-4-yl)-D-norleucine ethyl ester
[0511]
[0512] To a mixture of 4,6-dichloropyrimidine (15.0 g, 101 mmol) and D-norleucine ethyl ester (21.7 g, 111 mmol) in dioxane (440 mL) was added dropwise N, N'- Diisopropylethylamine (38.6 mL, 222 mmol). The mixture was stirred overnight at 65°C and then at 80°C for 4 hours. After cooling to room temperature, the mixture was evaporated under reduced pressure. The residue was diluted with water and extracted with ethyl acetate. The separated organi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com