Method for preparing L-iditol
A technology of iditol and sorbitol, applied in the field of preparing high-purity L-iditol
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
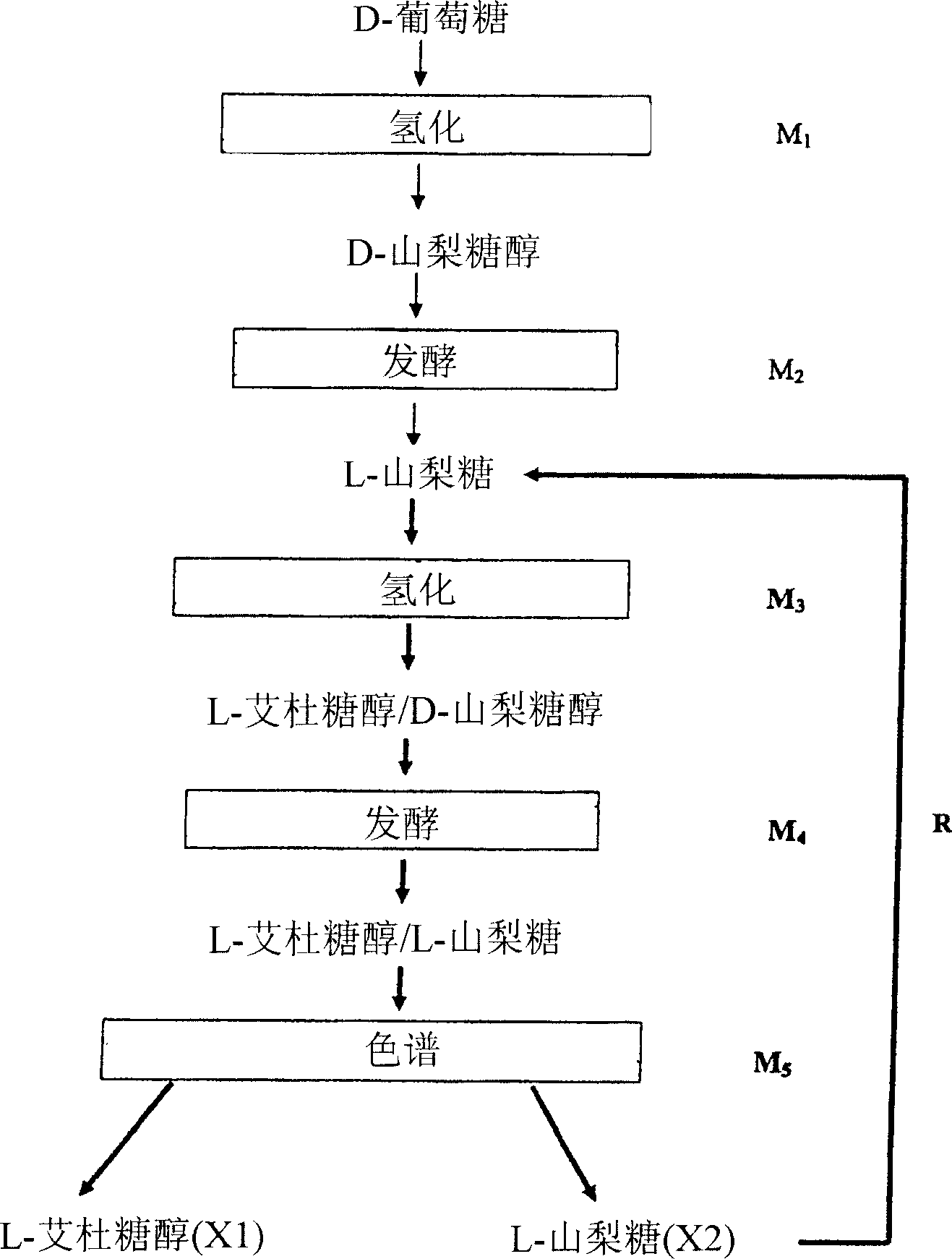
[0077] A mixture containing 45% L-sorbose and 55% L-iditol was chromatographed. In fact, this mixture contains very small amounts of other sugars and polyols, about 2%. Using Gluconobacter oxidans ATCC 19357 to ferment a mixture of D-sorbitol and L-iditol obtained by hydrogenation of a 40% L-sorbose solution and obtained. Hydrogenation using Raney nickel at substantially neutral pH yielded a slurry containing 0.2% residual reducing sugars and substantially equal amounts of D-sorbitol and L-iditol. After fermentation, the slurry was purified by filtration, then decolorized on carbon pellets, and finally desalted on ion exchange resins, followed by concentration to 50% solids.
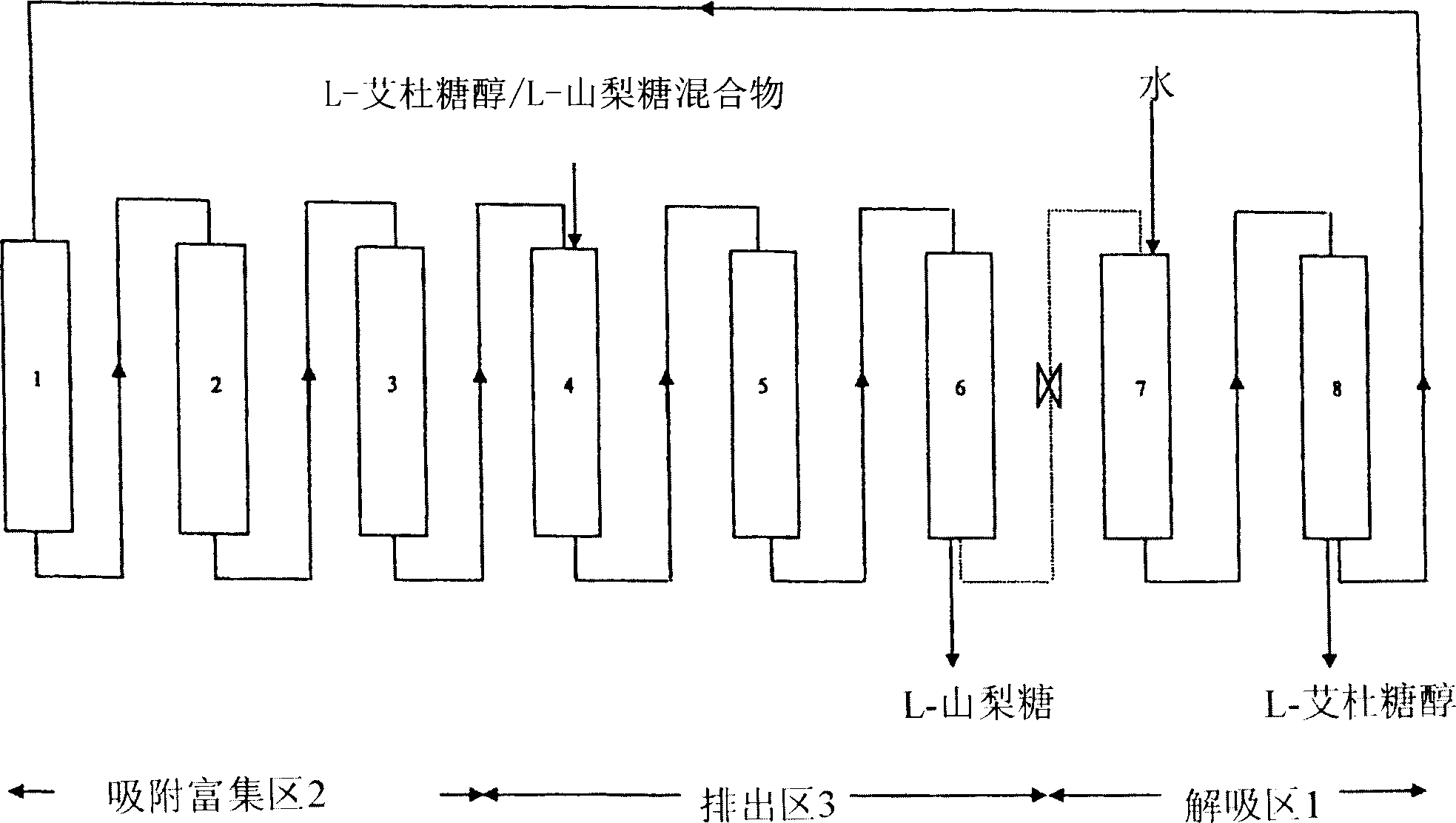
[0078] as in US Patent 4,422,881 figure 2 As shown, the chromatographic unit contains 8 columns or stages, each of 200 liters, with a strong ionic resin-type sorbent in the form of calcium and of fine and uniform particle size (0.2-0.3mm) (PCR732 from Purolite) , the content of this patent is incorp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com