Vitamine U, belladonna and aluminiium dispersible tablet and its preparation process
A technology of dispersible tablets and belladonna, applied in the field of medicine, can solve the problems of affecting the rapid effect of the drug, slow disintegration speed, long time for relieving stomach pain, gastric acid, and stomach distension, etc., and achieves inhibition of smooth muscle spasm and disintegration speed. Enhanced, fast-acting results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
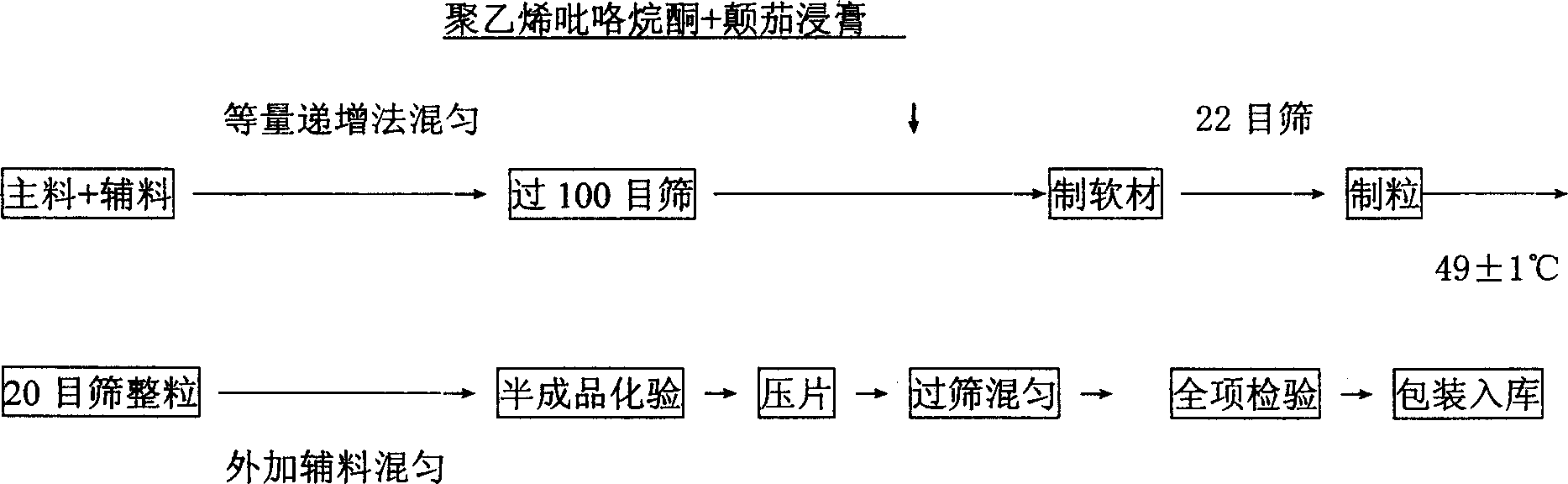
[0047] Take 50g vitamin U II, 140g aluminum hydroxide, 10g belladonna extract (calculated as atropine sulfate, not less than 0.15mg), 50g starch, 30g microcrystalline cellulose, 25g sodium carboxymethyl starch, 2g talcum powder, hard 2g magnesium fatty acid is made into 1000 tablets;
[0048] The preparation process is:
[0049] (1) Crush the raw materials and auxiliary materials separately, and pass through a 100-mesh sieve; weigh vitamin U II, aluminum hydroxide, belladonna extract and appropriate amount of starch according to the prescription, mix and absorb, and sieve and mix evenly;
[0050] (2) Take the prescription amount of vitamin U II, aluminum hydroxide and the above belladonna extract and mix them evenly by equal increasing method;
[0051] (3) Mix the mixed main medicine and internal auxiliary materials uniformly by equal increment method;
[0052] (4) The above mixture is made into a soft material with 10% starch slurry, and granulated with a 20-mesh screen; the wet ...
Embodiment 2
[0055] Take 50g vitamin U II, 140g aluminum hydroxide, 10g belladonna extract (calculated as atropine sulfate, not less than 0.15mg), 55g starch, 25g microcrystalline cellulose, 15g sodium carboxymethyl starch, plus carboxymethyl 5g of sodium starch, 2g of talc and 2g of magnesium stearate are made into 1000 tablets;
[0056] The preparation process is the same as in Example 1.
Embodiment 3
[0058] Take 50g vitamin U II, 140g aluminum hydroxide, 10g belladonna extract (calculated as atropine sulfate, not less than 0.15mg), 60g starch, 20g microcrystalline cellulose, 10g sodium carboxymethyl starch, plus carboxymethyl 5g sodium starch and 3g magnesium stearate are made into 1000 tablets;
[0059] The preparation process is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com