High throughput biological indicator reader
A biological indicator and reader technology, applied in sanitary equipment for toilets, biochemical equipment and methods, and microorganism measurement/inspection, etc. The effect of high throughput and convenient storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
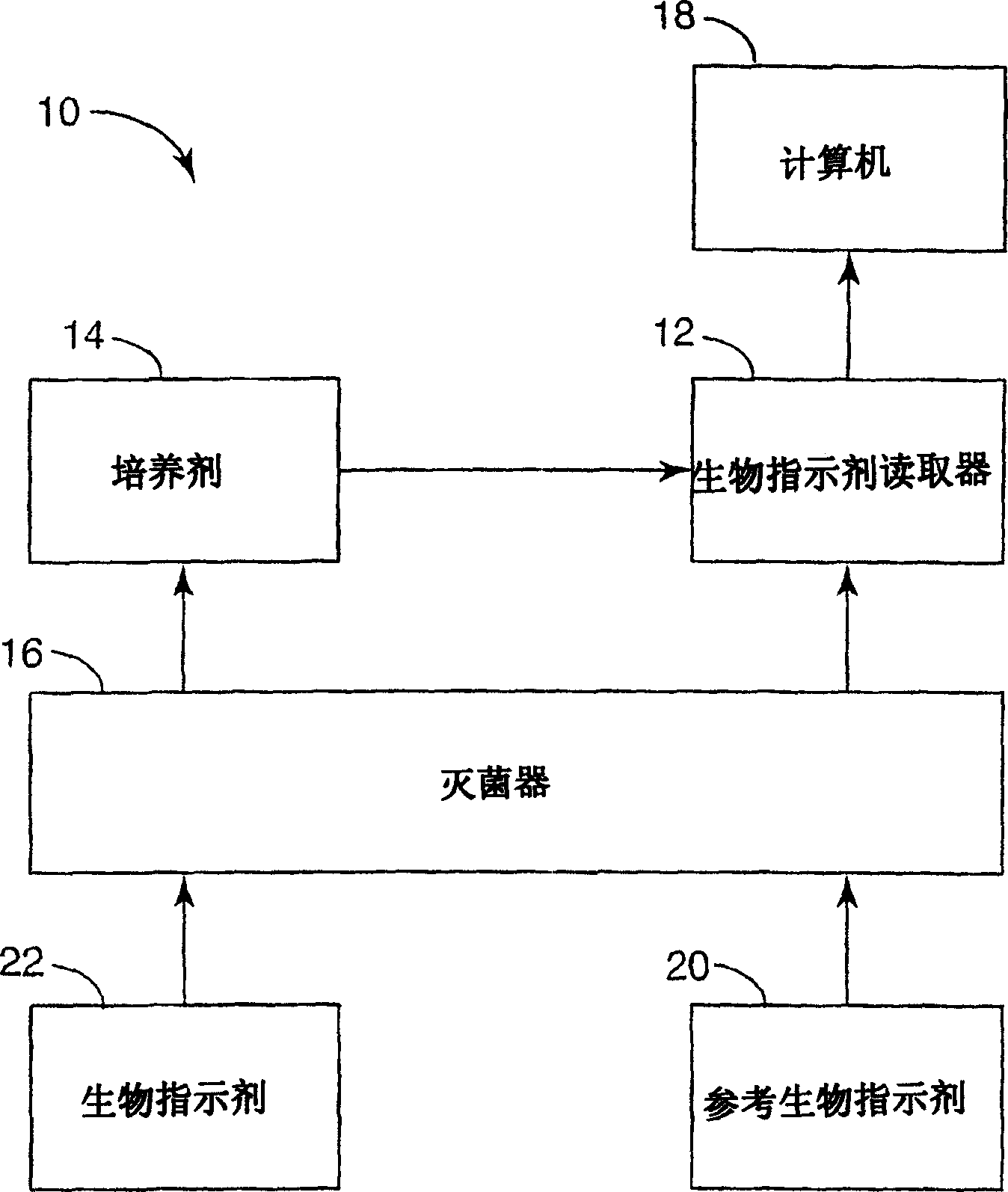
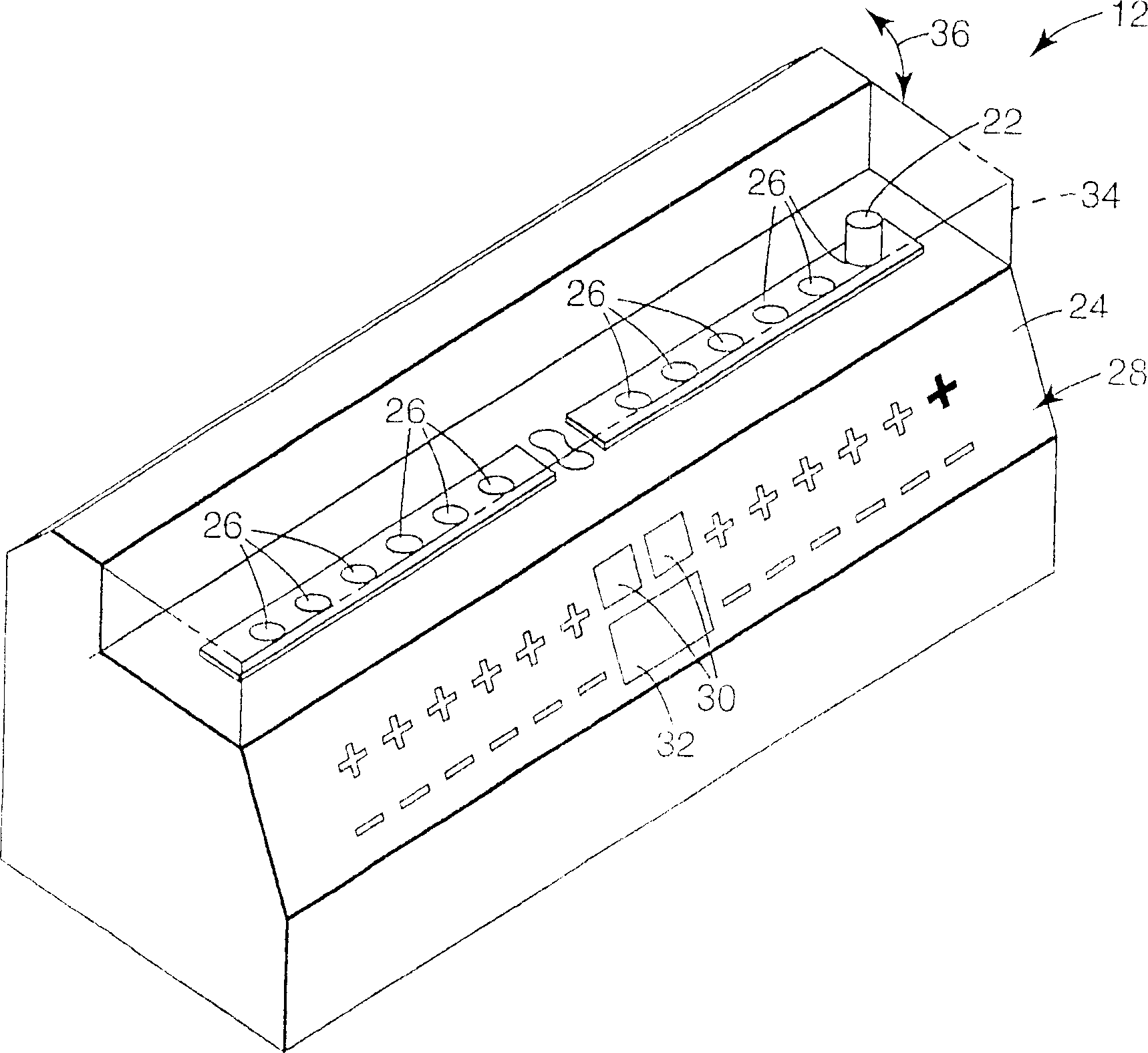
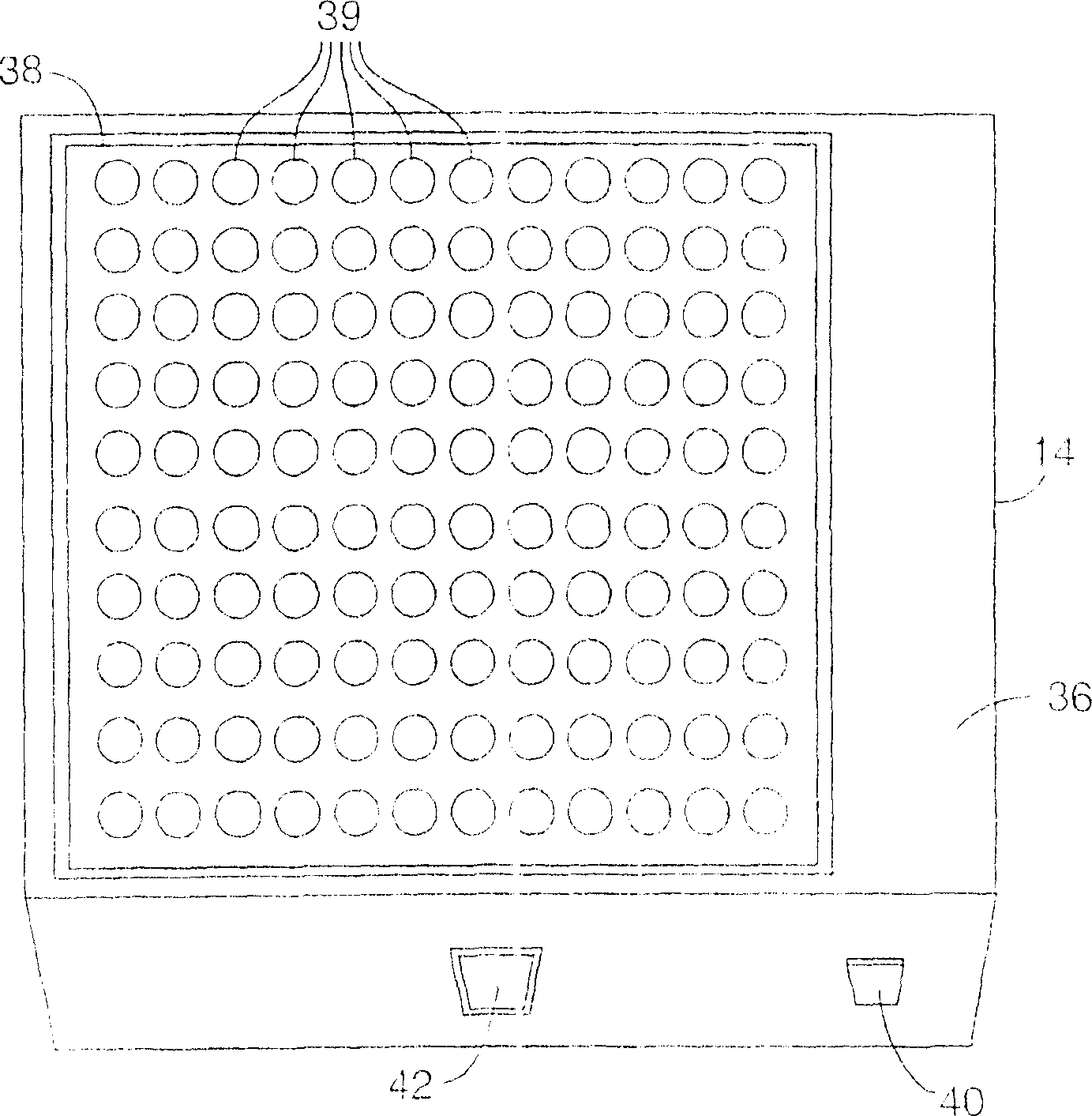
[0028] figure 1 is a block diagram illustrating a system 10 for analysis of biological indicators. Such as figure 1 As shown in , system 10 may include biological indicator reader 12 , biological indicator incubator 14 , sterilizer 16 and computer 18 . Reader 12 processes reference biological indicator 20 to obtain an initial baseline fluorescence measurement for later evaluation of biological indicator 22 to detect proper sterilization. In general, system 10 supports high volume and high throughput in reading out biological indicators 22 to detect fluorescence and thereby determine the efficacy of a sterilization cycle.
[0029]System 10 may be used in healthcare facilities to monitor sterilization efficacy over large sterilization loads. Alternatively, system 10 may be used to estimate the sterilization efficacy of equipment, instruments, medical devices, implants, and other items before such items are commercially released from a manufacturing facility, among other thing...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com