Recombinant a human peptide production method
A technology of Xili peptide and Xili, which is applied in the field of preparation of biologically active peptides, can solve the problems of low yield and complicated process steps, and achieve the effects of high expression rate, simplified production process, and improved production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] 1. Selection of expression vector
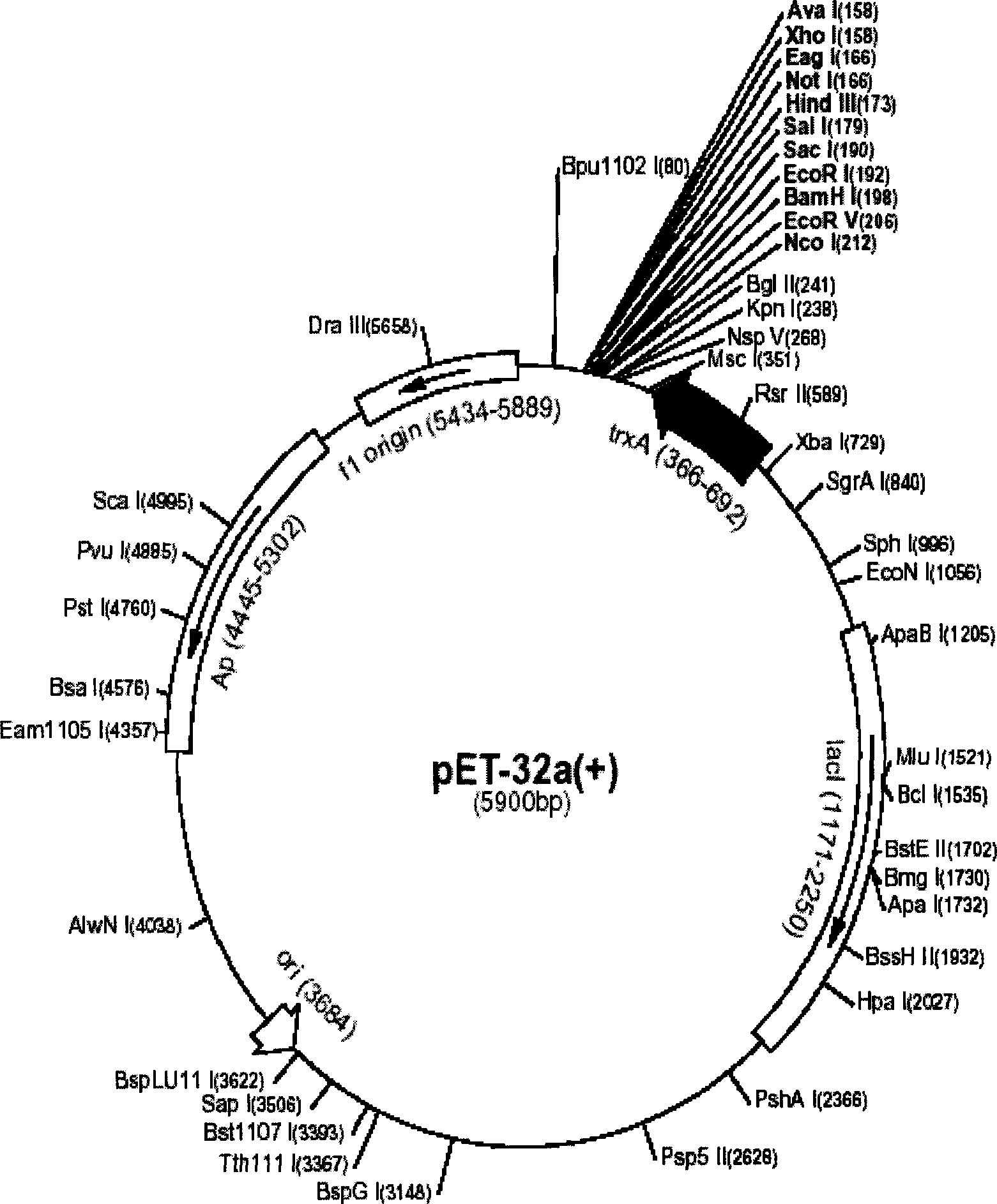
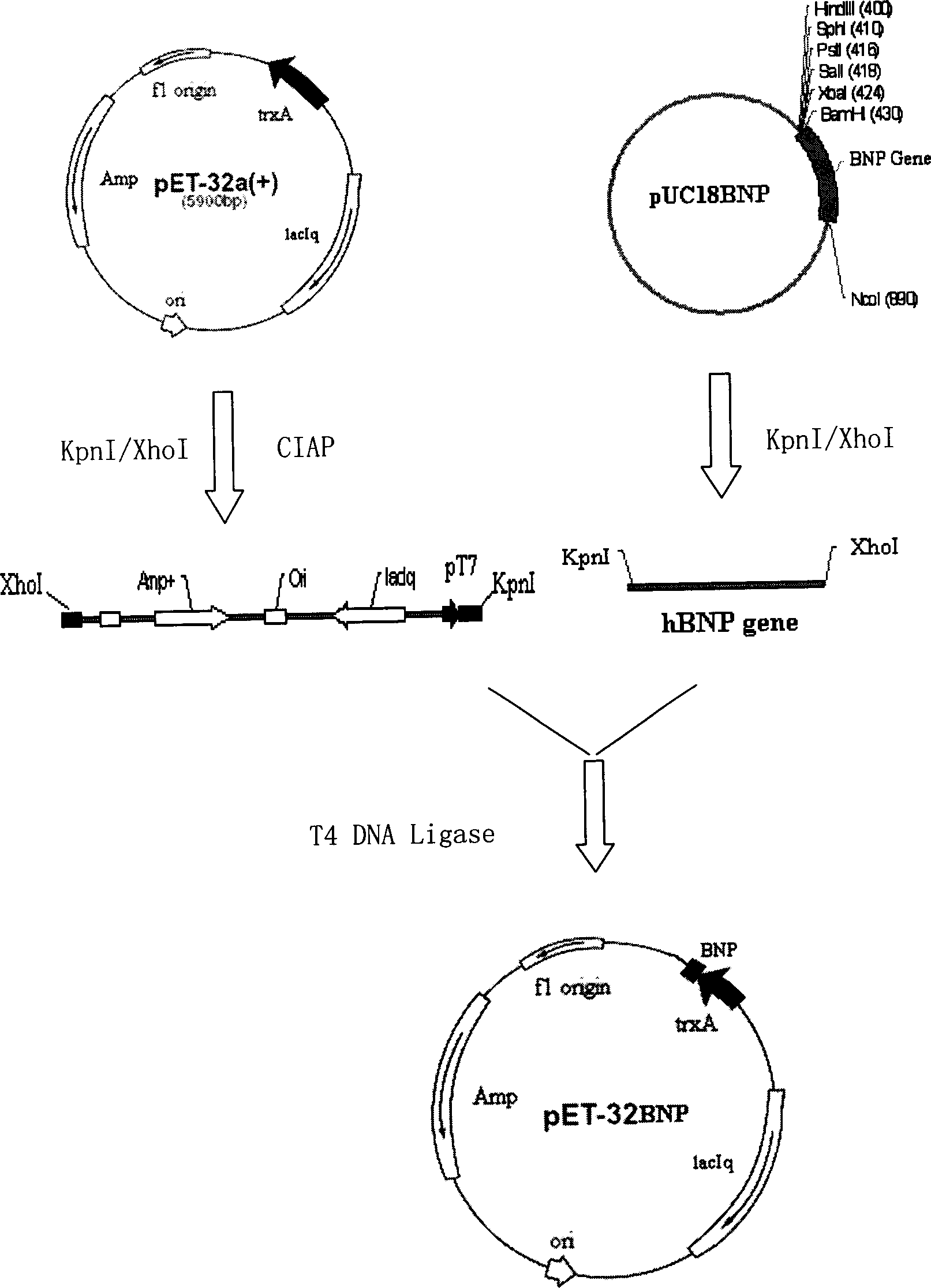
[0060] The pET series expression vector pET32a from Novagen was selected as the expression vector. The pET-32a is an Escherichia coli expression vector system for high-efficiency fusion expression of proteins and polypeptides. The target protein or polypeptide is thioredoxin (Trx ·Tag TM ) as a fusion partner. (See attached for map figure 1 , figure 2 ).
[0061] 2. Gene design and synthesis
[0062] According to the human nosire peptide DNA coding sequence reported in the literature (the sequence can be changed according to the principles of universality and degeneracy of the genetic code, but the amino acid composition and arrangement are not changed), the coding mature human BNP sequence is designed, such as: 5'_AGC CCT AAA ATG GTA CAG GGT TCT GGT TGC TTC GGT CGT AAA ATG GAC CGTATC AGC TCT TCC AGC GGT CTG GGT TGC AAA GTA CTG CGT CGT CAC TAA_3'. At the same time, add Kpn recognition sequence GGTACC and enterokinase (Enterokin...
Embodiment 2
[0078] 1. Selection of expression vector
[0079] The pTDa of our laboratory was selected as the expression vector (see attached Figure 6 )
[0080] 2. Gene design and synthesis
[0081] According to the preference principle of Escherichia coli for the use of genetic code, the coding sequence of mature human nosire peptide was designed: 5'_AGC CCT AAA ATG GTA CAG GGT TCT GGT TGC TTC GGT CGT AAA ATG GACCGT ATC AGC TCT TCC AGC GGT CTG GGT TGC AAA GTA CTG CGT CGT CAC TAA (the sequence can be changed according to the principle of universality and degeneracy of the genetic code, but the amino acid composition and arrangement will not be changed). Add EcoRI recognition sequence GAA TTC and thrombin (Thrombin) restriction site (-LVPR-X) coding sequence CTG GTG CCT CGT at the 5'-end of the sequence at the same time; add the recognition of Sal I at the 3'-end The sequence GTC GAC thus forms the final design sequence as follows: 5'_GAA TTC CTG GTG CCT CGT AGC CCTAAA ATG GTA CAG GGT ...
Embodiment 3
[0090] According to the physicochemical property determination of the rhBNP of embodiment 1 or 2 gained
[0091] 1. Determination of the N-terminal 15 amino acid sequences
[0092] The recombinant human nosire peptide obtained by the above two methods was subjected to N-terminal determination, Ser-Pro-Lys-Met-Val-Gln-Gly-Ser-Gly-Cys-Phe-Gly-Arg-Lys-Met
[0093] 2. Molecular weight mass spectrometry
[0094] The FAB method was used to determine the molecular weight of the recombinant human nosiritide obtained by the two methods was 3462, which was consistent with the theoretical prediction.
[0095] 3. Determination of biological activity
[0096] Use Phenylephrine to bind with its receptors on blood vessels to produce a vasoconstrictor effect, increase the resting tension of blood vessels and maintain it at a stable level, and then use rhBNP to combine with its natriuretic peptide receptors on blood vessels to produce a vasoconstrictive effect on rabbit chest The vasodilato...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com