Kit and application based on recombinant protein of Riemerella anatipestifer omph truncated
A technology of Riemer's duckweed and recombinant protein, applied in the field of bioengineering, can solve the problems of complex bacterial antigen composition, difficult application, low sensitivity, etc., and achieve the effects of wide market application prospect, low production cost and stable performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] The preparation of embodiment 1 Riemerella anatipestifer OmpH truncated recombinant protein
[0057] 1. Main experimental materials
[0058] Plasmid T-Vector pMD19(simple), Max DNA Polymerase was purchased from Dalian Bao Biological Engineering Co., Ltd.; prokaryotic expression plasmid pET32a(+), product of Novagen; clone host bacteria E.coli DH5α, expression host bacteria E.coli BL21(DE3) and RA-CH-1 The strains were provided by the Poultry Disease Research Center of Sichuan Agricultural University.
[0059] 2 Experimental methods
[0060] 2.1 Cloning of RA OmpH truncated gene
[0061] 2.1.1 Primer design
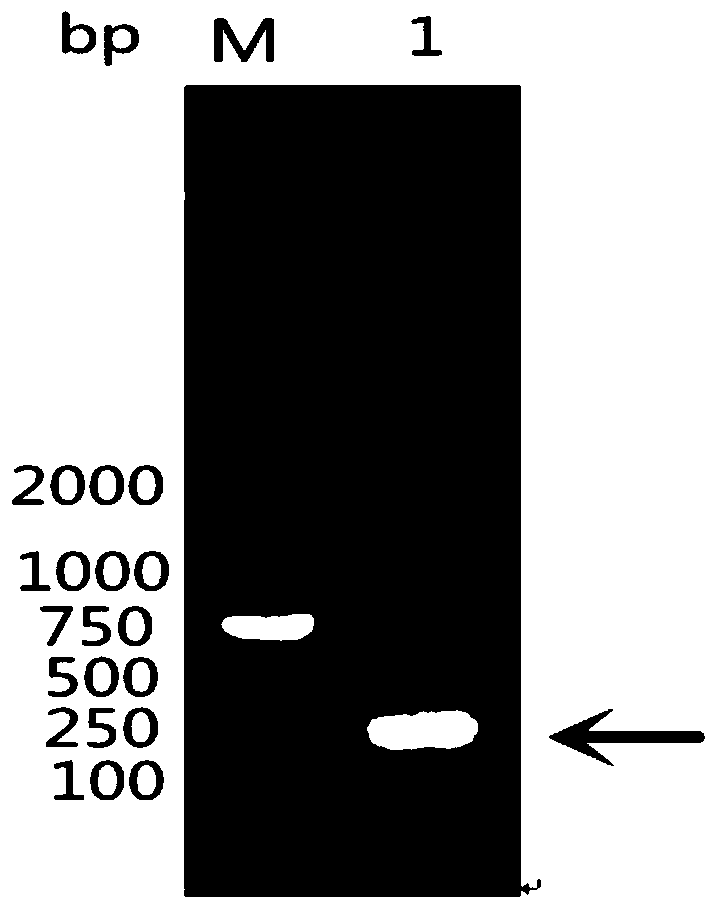
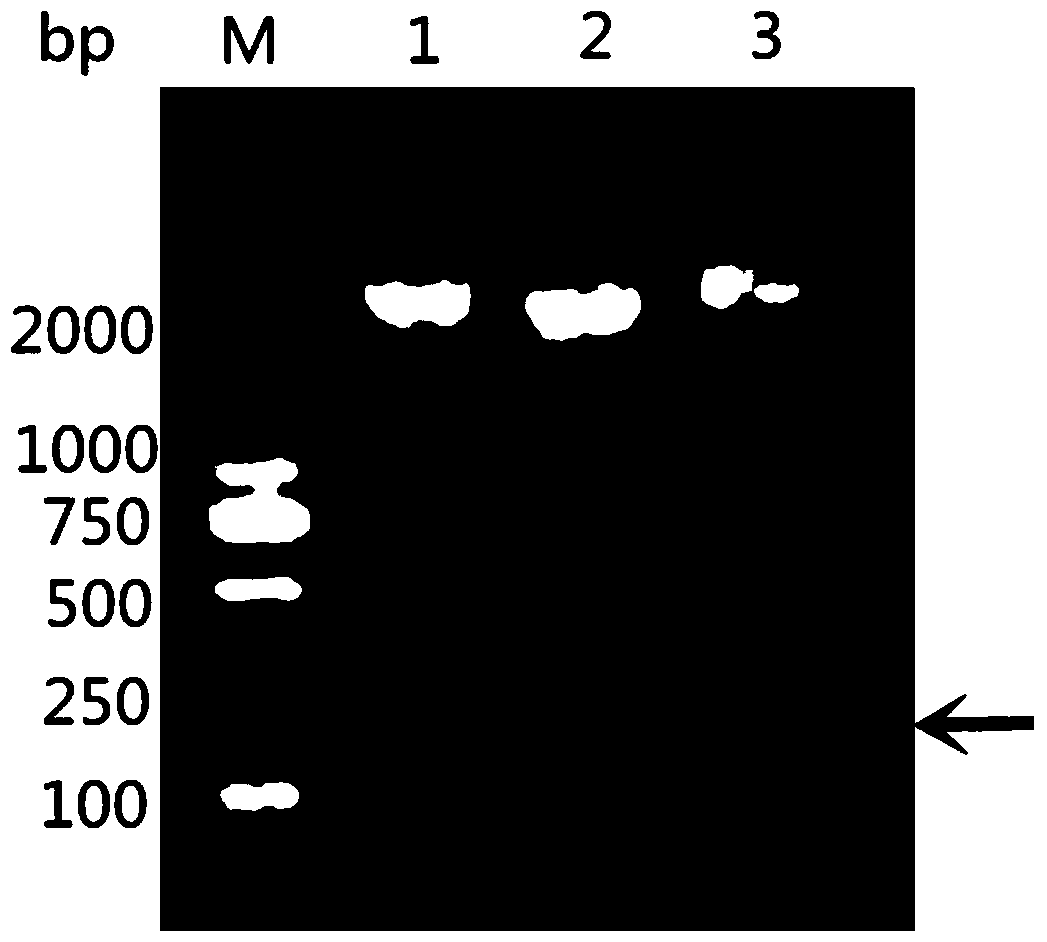
[0062] According to the existing RA-CH-1OmpH gene sequence in GenBank, the main antigenic domain (81aa-151aa and named as OmpHM) was selected, and a pair of primers were designed using Primer Premier5.0 software. Upstream primer as shown in SEQ ID NO:3: 5'-CGC GGATCC GAGGCTCAGAGAACTGCT-3' (the underlined part is the BamH Ⅰ site); the downstream primer shown ...
Embodiment 2
[0144] Embodiment 2 Purification of Riemerella anatipestifer OmpH truncated recombinant protein
[0145] After the expression product containing the OmpH truncated recombinant protein of Riemerella anatipestifer prepared in Example 1 was collected from the bacteria, ultrasonically disrupted, and supernatant collected, the recombinant protein was purified by nickel agarose gel affinity chromatography. OmpH truncated recombinant protein. The UV curve of the protein sample after column purification showed three peaks, peak 1 was the breakthrough peak, peak 2 was the elution peak of 50 mmol imidazole, and peak 3 was the elution peak of 100 mmol imidazole. Collect different concentrations of imidazole elution peaks simultaneously, carry out SDS-PAGE electrophoresis, check purity and concentration, the result shows: only contain a large amount of high-purity OmpH truncated recombinant proteins in the 100mmol imidazole elution peak ( Figure 8 ). After the purified OmpH truncated r...
Embodiment 3
[0146] Western-blot (western blot) of embodiment 3 Riemerella anatipestifer OmpH truncated recombinant protein
[0147] 1. Experimental method
[0148] The recombinant protein containing the OmpH truncated fragment of Riemerella anatipestifer obtained in Example 1 was used as a probe for detecting the serum antibody of Riemerella anatipestifer.
[0149] 1SDS-PAGE gel preparation
[0150] 1.1 Preparation of 12% separating gel
[0151] Deionized water 1215ul, 1.5M Tris-HCl (PH=8.8) 950ul, 10% SDS 37.5ul, 10% AP37.5ul, TEMED 1.5ul, 30% acrylamide 1.5ml.
[0152] 1.2 Preparation of 5% stacking gel
[0153] Deionized water 700ul, 1.0M Tris-HCl (PH=6.8) 125ul, 10% SDS 10.0ul, 10% AP 10.0ul, TEMED 1.0ul, 30% acrylamide 165ul.
[0154] 2 Processing of protein samples
[0155] Add an appropriate amount of SDS-PAGE protein loading buffer (0.5M Tris-HCl (PH=6.81.2ml), glycerin 1ml, deionized water 4.8ml, 10% SDS 2.0ml, 0.1% BPB 0.5ml) to the protein sample . 10000r / min 10min after...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com