High-expression vector for animal cells
A technology for expressing vectors and animal cells, applied in vectors, using vectors to introduce foreign genetic material, microorganisms, etc., can solve problems such as low productivity of recombinant protein expression systems and problems with the stability of introduced genes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
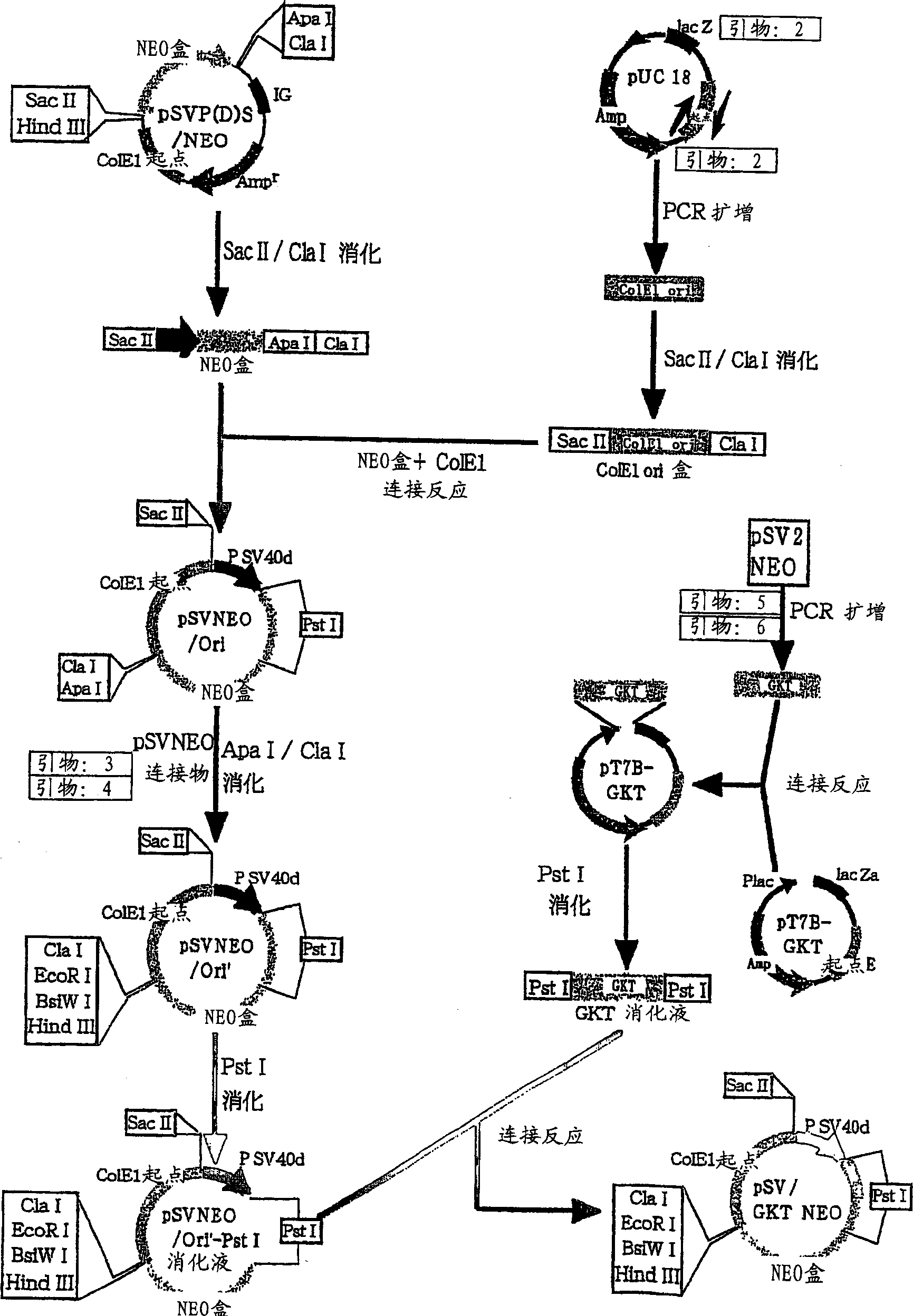
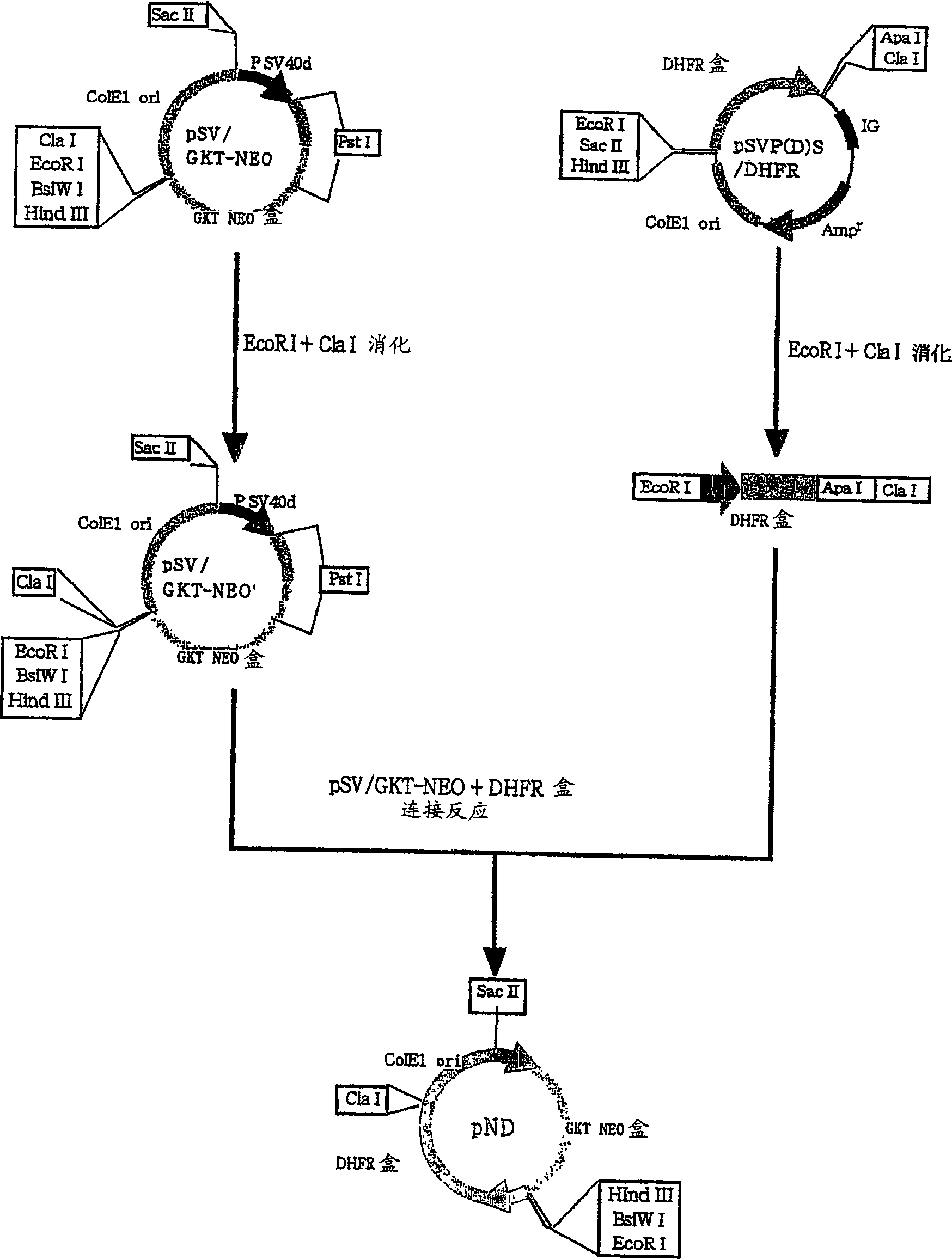
[0067] Embodiment 1: Construction of the expression vector of the present invention
[0068] (1) Preparation of neomycin-resistant vector [pSV / GKT-Neo]
[0069] The NEO vector [pSV / GKT-Neo] has the Col E1-Ori (Col E1 ori) and neomycin phosphotransferase (NPT) gene cistrons derived from plasmid pUC18 (Gene, 33, p. 103, 1985). Col E1-Ori was amplified from plasmid pUC18 by the PCR method using primers having the base sequences shown in SEQ ID NO.1 and 2. PCR was carried out under the following conditions. The PCR kit uses the kit sold by Takarajiu, and the 10-fold concentrated reaction buffer and dNTP solution are all accessories of the kit. First, add 10-fold concentrated reaction buffer, dNTP solution, 1 μl of 100 μM primers recorded in SEQ ID NO.1 and 2, 1 μl template DNA, 0.5 μl Pyrobest DNA polymerase (5 U / μl) to 36.5 μl sterile distilled water, After mixing thoroughly, the PCR reaction was performed by Takara Thermal Cycler PERSONAL. That is, heat treatment at 94°C for...
Embodiment 2
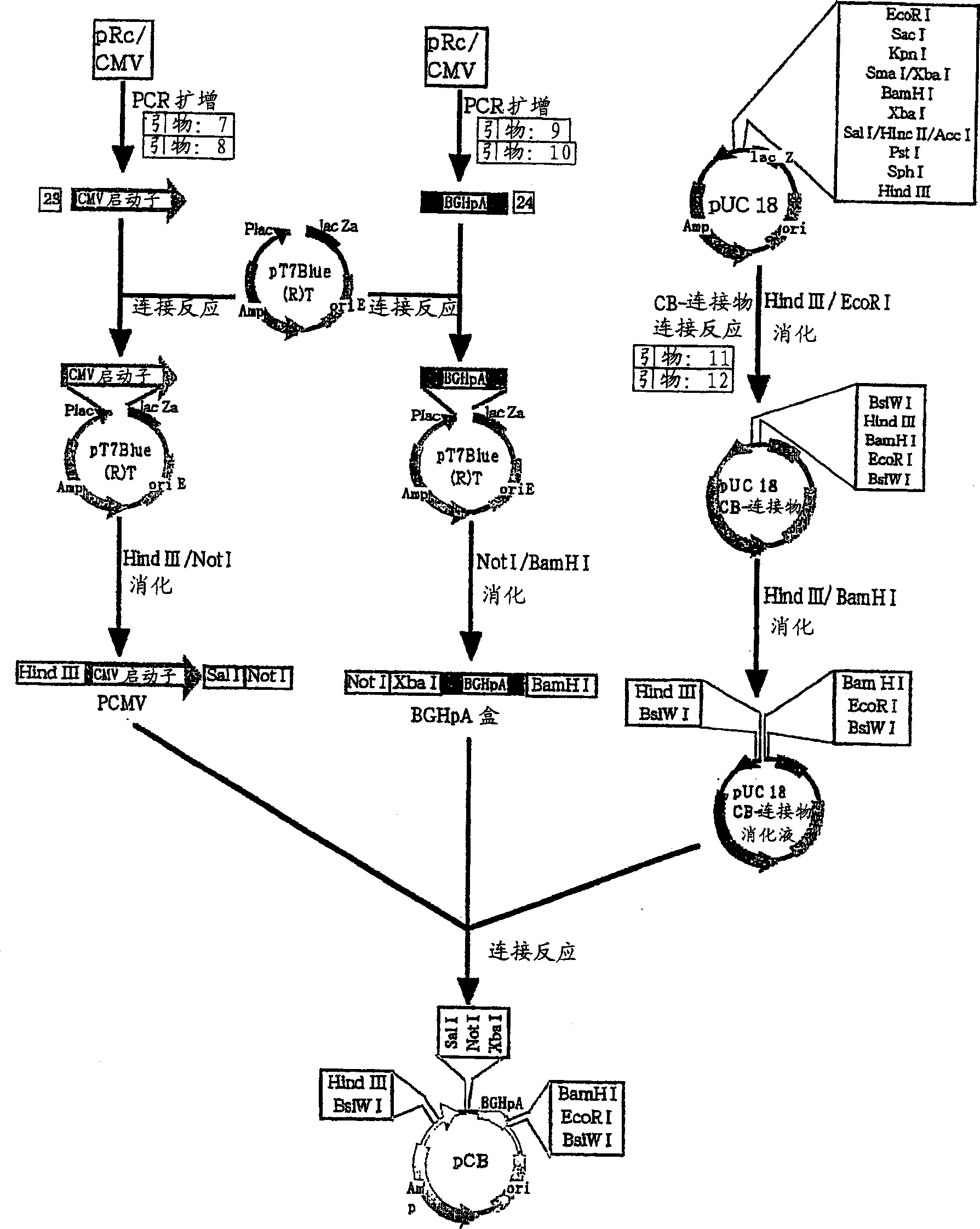
[0090] Example 2: Production test of human granulocyte colony-stimulating factor carried out using the expression vector of the present invention
[0091] As the target substance for the transfection of human granulocyte colony-stimulating factor (hG-CSF) by the expression vector pNOS-GKT2B-R and the establishment of G418-resistant early clones, hG-CSF, a kind of colony-stimulating factor, was selected. The protein has sugar chains, and the recombinant protein in normal form cannot be obtained in the production of Escherichia coli or yeast as the host.
[0092] (1) Preparation of hG-CSF expression vector [pNOS-GKT2B-R / hG-CSF]
[0093] Using primers with the base sequences shown in SEQ ID NO.22 and 23, by using the PCR method using LATagDNA polymerase, amplified from human spleen cDNA, developed, separated and purified by agarose gel to obtain about 600 Base pair, fragment of cDNA containing hG-CSF (hG-CSF fragment; 13 ng / μl). It was connected with pT7Blue(R) by ligation reac...
Embodiment 3
[0102] Example 3: Gene Amplification of G418 Resistant Cell Lines
[0103] Among the G418-resistant cell lines, a clone whose hG-CSF production was 77 μg / ml / 3 days was subcultured and stabilized, and then gene amplification was performed by MTX. Start with 15 nM MTX for 2 weeks, then 60 nM MTX for 2 weeks, then 250 nM MTX for 2 weeks, then 1 μM MTX for 2 weeks At this time, the production level of hG-CSF rose to 117 μg / ml / 3 days relative to the culture medium. The concentration of MTX used in gene amplification is usually in multiple stages between 10nM-10μM, and the final concentration is often 10μM. However, due to the toxicity to cells, exposure to high concentrations has problems in establishing stable recombinant cell lines. Therefore, high productivity can be achieved at a low concentration of MTX, which also becomes an important evaluation criterion, and in this experiment, the maximum was 1 μM. The period of MTX exposure including usual selection is usually 6-12 mont...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com