Young schistosomiasis cell style vaccine for preventing from schistosomiasis disease
A technology for schistosomiasis and schistosomiasis, which is applied in the field of schistosomiasis cell-based vaccines, can solve the problems of impracticality, unsafe cercariae live vaccine consumption, poor resistance to attacking infected worms, etc., and achieves easy production and preparation , Overcome the large amount of materials consumed and the effect of overcoming insecurity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
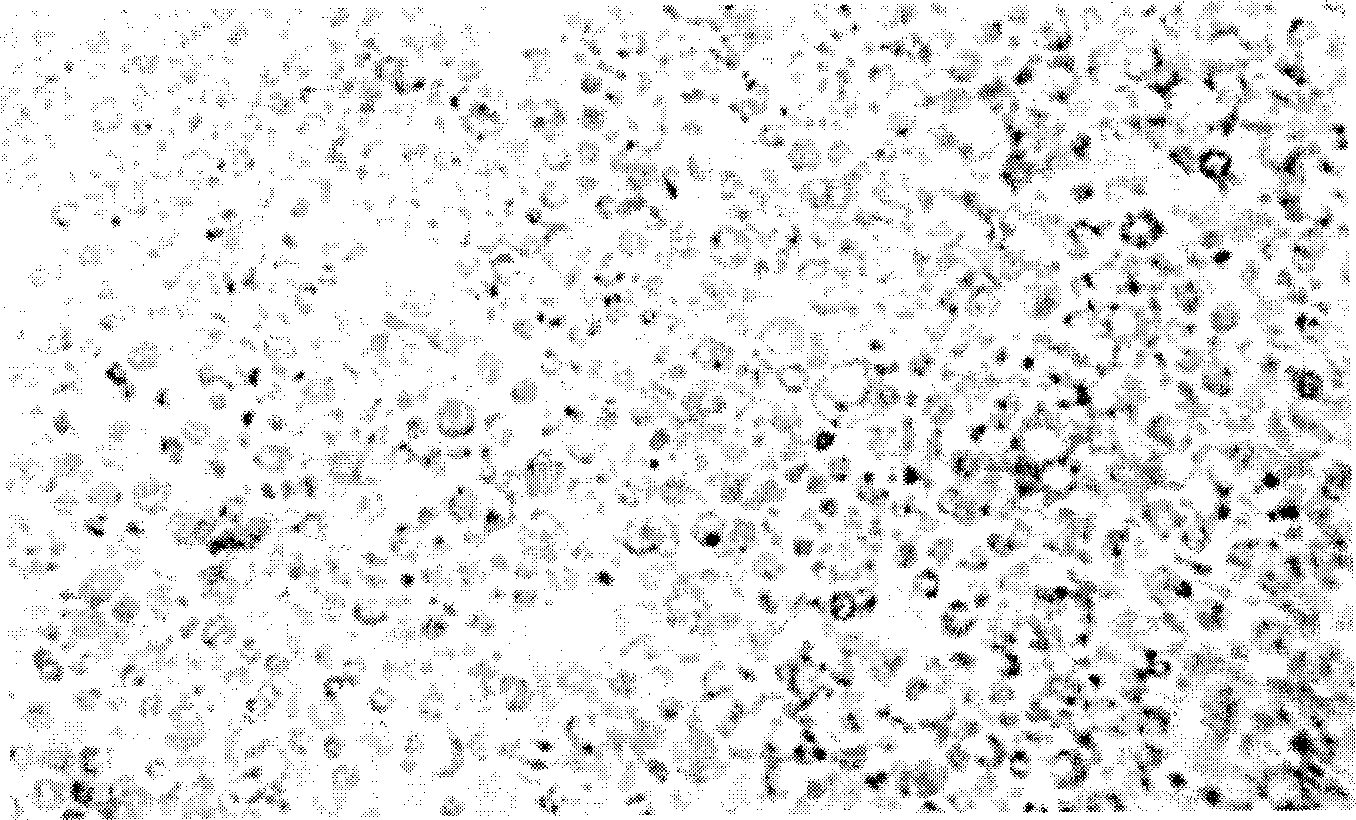
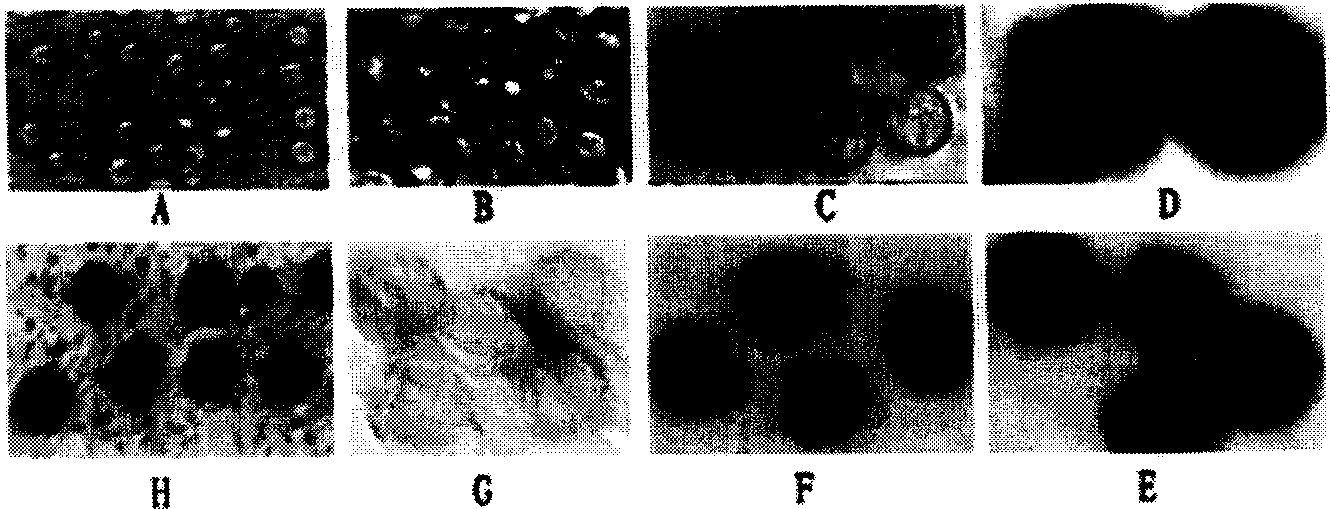
[0016] 1. Preparation of Pediatric Worm Cell Vaccine
[0017] The cercariae of Schistosoma japonicum were used to perfuse the healthy rabbits on the 14th to 24th day after the abdomen was heavily infected. After the worms were collected and washed, the worms were quickly chopped under aseptic conditions, and 10 times the worms containing 0.25% trypsin and 0.02% EDTA digestion solution was digested for 30min, the supernatant was aspirated, and Ca 2+ , Mg 2+ The D-Hank's solution was used to stop the digestion; the obtained cells were centrifuged at 2000rpm×5min, washed with D-Hank's repeatedly for 3 times, the non-cellular components in the supernatant were discarded, and then a small amount of PBS or D-Hank's solution was added for cell counting , and set the volume to 1.6×10 7 / ml, used as the immunization dose concentration. The living cells of Schistosoma japonicum hepatic stage juveniles were obtained, and the somatic cells of Schistosoma japonicum were obtained accordi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com