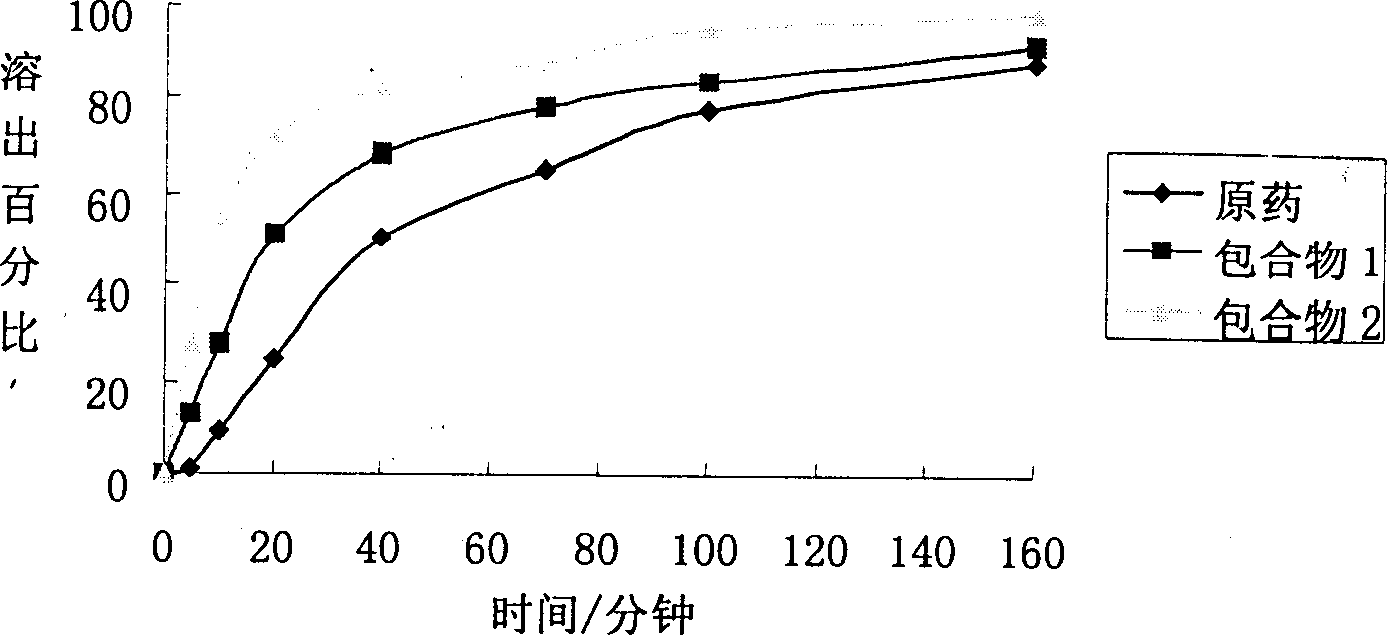
Astragaloside cyclodextrin clathrate, its prepn. and preparing mehtod
The technology of cyclodextrin inclusion complex and astragaloside IV is applied in the directions of non-active components of polymer compounds, pharmaceutical formulations, pharmaceutical combinations, etc., and can solve the problems of limited wide use, difficulty in preparing injections, low oral bioavailability, and the like, Achieve the effect of expanding application prospects, enriching formulation varieties, improving solubility and bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Preparation of astragaloside β-cyclodextrin inclusion compound
[0042] Weigh 5.0g of astragaloside IV, add 1250ml of ethanol and heat to dissolve; weigh another 7.2g of β-cyclodextrin, add 360ml of distilled water, heat and stir to dissolve; slowly add astragaloside IV ethanol solution dropwise while stirring, and keep the temperature at 50°C , continue to stir for half an hour, stop heating, let it gradually drop to room temperature, continue to stir until the remaining 1 / 5 of the solution, and dry under reduced pressure to obtain. The clathrate solubility reaches 82.5μg / ml.
Embodiment 2
[0044] Preparation of astragaloside β-cyclodextrin inclusion compound
[0045] Weigh 5.0 g of astragaloside IV, add 950 ml of methanol and heat to dissolve; weigh another 7.2 g of β-cyclodextrin, add 360 ml of distilled water, and heat to dissolve; slowly add astragaloside IV methanol solution dropwise under ultrasound, maintaining the temperature at 40 °C, Continue to sonicate for half an hour, stop heating, let it drop to room temperature gradually, continue to sonicate until the remaining 1 / 5 of the solution, and dry under reduced pressure to obtain the product. The clathrate solubility reaches 80.9μg / ml.
Embodiment 3
[0047] Preparation of astragaloside β-cyclodextrin inclusion compound
[0048] Weigh 5.0g of astragaloside IV, add about 1250ml of ethanol and heat to dissolve; weigh another 14.4g of β-cyclodextrin, add 720ml of distilled water, heat and stir to dissolve; slowly add astragaloside IV ethanol solution dropwise while stirring, and maintain the temperature at 50 ℃, continue to stir for 1 hour, stop heating, let it gradually lower to room temperature, continue to stir until the remaining 1 / 10 of the solution, and dry under reduced pressure to obtain. The clathrate solubility reaches 195.6μg / ml.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Solubility | aaaaa | aaaaa |
| Solubility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com