Preparation method of red or green long afterglow glass using zinc borate as substrate
A technology of zinc borate and long afterglow, applied in the field of preparation of red and green long afterglow glass, can solve the problems of short afterglow, high power of femtosecond laser, high price, etc., achieve low melting temperature, guaranteed afterglow performance, and reduce construction cost and the effect of production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Each component of this glass composition is weighed by the following mole percentages:
[0015] ZnO: 69.00%; B 2 o 3 : 30.99%;
[0016] MnCO 3 : 0.01%.
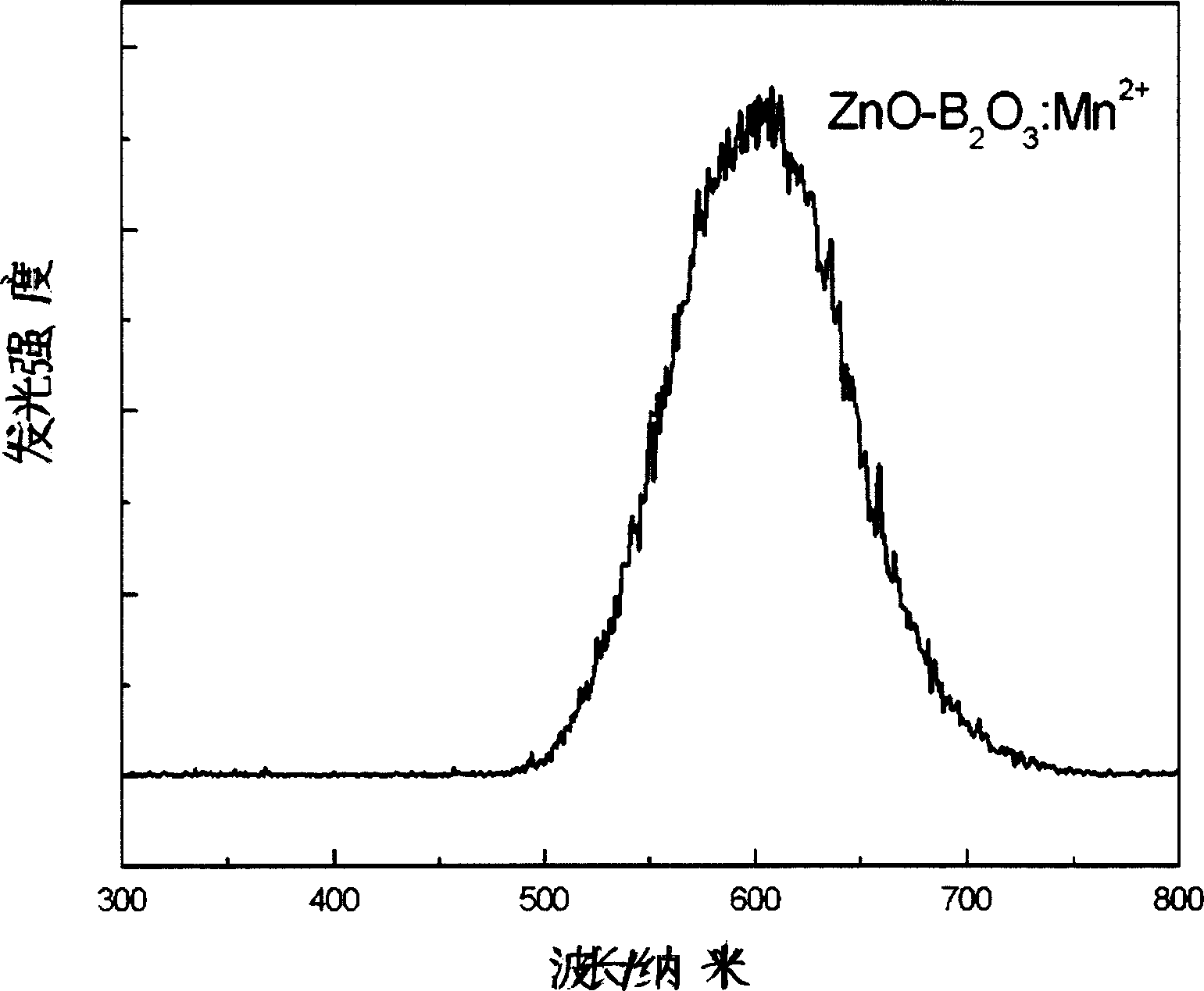
[0017] The raw material components accurately weighed according to the composition ratio were finely ground and mixed, and then put into a crucible, and carbon powder was placed in the furnace as a reducing atmosphere, and the temperature was kept at 900°C for 5 hours. After the glass is out of the furnace, it is poured into a stainless steel mold for molding, and then annealed at 500°C for 4 hours in an air atmosphere, and then cooled to room temperature naturally to obtain a colorless and transparent long-lasting glass; after 20 minutes of irradiation with a 254nm ultraviolet lamp, the red afterglow of the glass Up to 12 hours.
Embodiment 2
[0019] Each component of this glass composition is weighed by the following mole percentages:
[0020] ZnO: 69.00%; B 2 o 3 : 30.99%
[0021] MnCO 3 : 0.01%.
[0022] The raw material components accurately weighed according to the composition ratio are finely ground and mixed, and then put into a crucible, and hydrogen gas is introduced into the furnace as a reducing atmosphere, and the temperature is kept at 1500°C for 0.5h. After the glass is out of the furnace, it is poured into a stainless steel mold for molding, and then annealed at 450°C for 5 hours in an air atmosphere, and then cooled to room temperature naturally to obtain a colorless and transparent long-lasting glass; after 20 minutes of irradiation with a 254nm ultraviolet lamp, the red afterglow of the glass Up to 12 hours.
Embodiment 3
[0024] Each component of this glass composition is weighed by the following mole percentages:
[0025] ZnO: 34.00% B 2 o 3 : 65.00%
[0026] MnCO 3 : 0.10% Zn: 0.90%
[0027] The raw material components will be accurately weighed according to the composition ratio, ground and mixed, put into a crucible, and kept at 1400°C for 1 hour in an air atmosphere. After the glass is out of the furnace, it is poured into a stainless steel mold for molding, and then annealed at 500°C for 4 hours in an air atmosphere, and then cooled to room temperature naturally to obtain a colorless and transparent long-lasting glass; after 20 minutes of irradiation with a 254nm ultraviolet lamp, the red afterglow of the glass Up to 12 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com