Method for preparing lithium magnesium silicate montmorillonite using salt lake brine water
A technology of magnesium lithium silicate, salt lake brine, applied in chemical instruments and methods, silicon compounds, inorganic chemistry, etc., can solve problems such as chemical composition imbalance, endanger the development of salt lake resources, etc. The effect of high added value of products and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
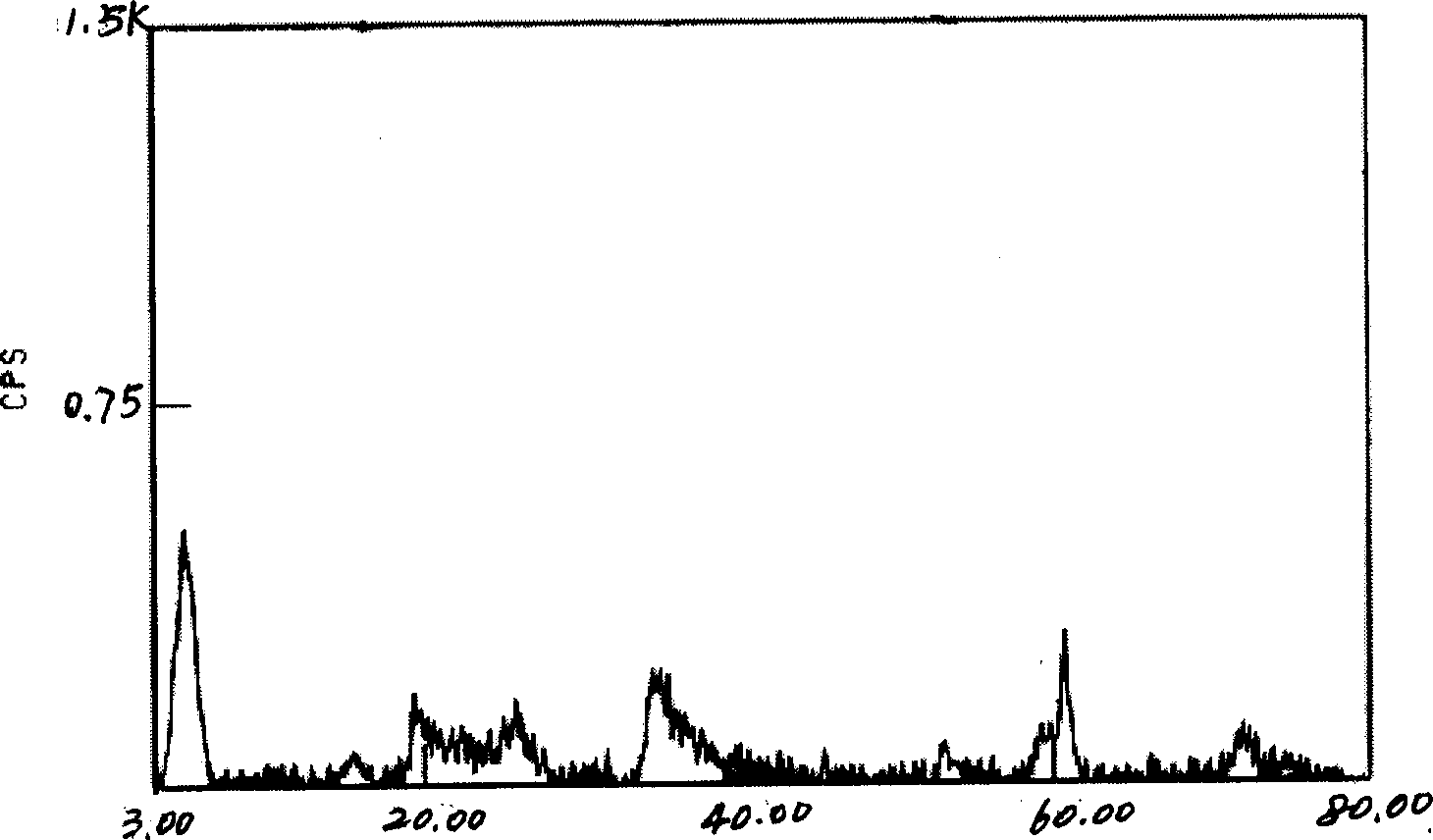
[0026] Get 1000g concentrated brine, its chemical composition weight percent is: LiCl 2.50%; NaCl 1.42%; KCl 0.18%; MgCl 2 29.13%; MgSO 4 3.75%; B 2 o 3 1.66%; H 2 O 61.37%; 2234.78g Na 2 SiO 3 9H 2 O was added to the concentrated brine of the salt lake, stirred to make it fully mixed, and NaOH solution was added to adjust the pH value of the mixture to 11, and 394.02g MgCl 2 ·6H 2 OH adjusts the ratio of the amount of magnesium, lithium and silicate in the mixed solution to Mg 2+ : Li + : Si 4+ =2.7:0.3:4; then the mixed solution was placed in a high-pressure reactor, reacted at 250°C for 6 hours, cooled, the gel-like product was taken out, washed by centrifugation, dried, and ground into a powder. The white powder sample obtained by XRD test is magnesium silicate hectorite clay. Its composition is:
[0027] Na 2 O 3.23%; Li 2 O 1.17%; MgO 28.39%; SiO 2 62.71%; H 2 O 4.70%. The recovery rate was 92%.
Embodiment 2
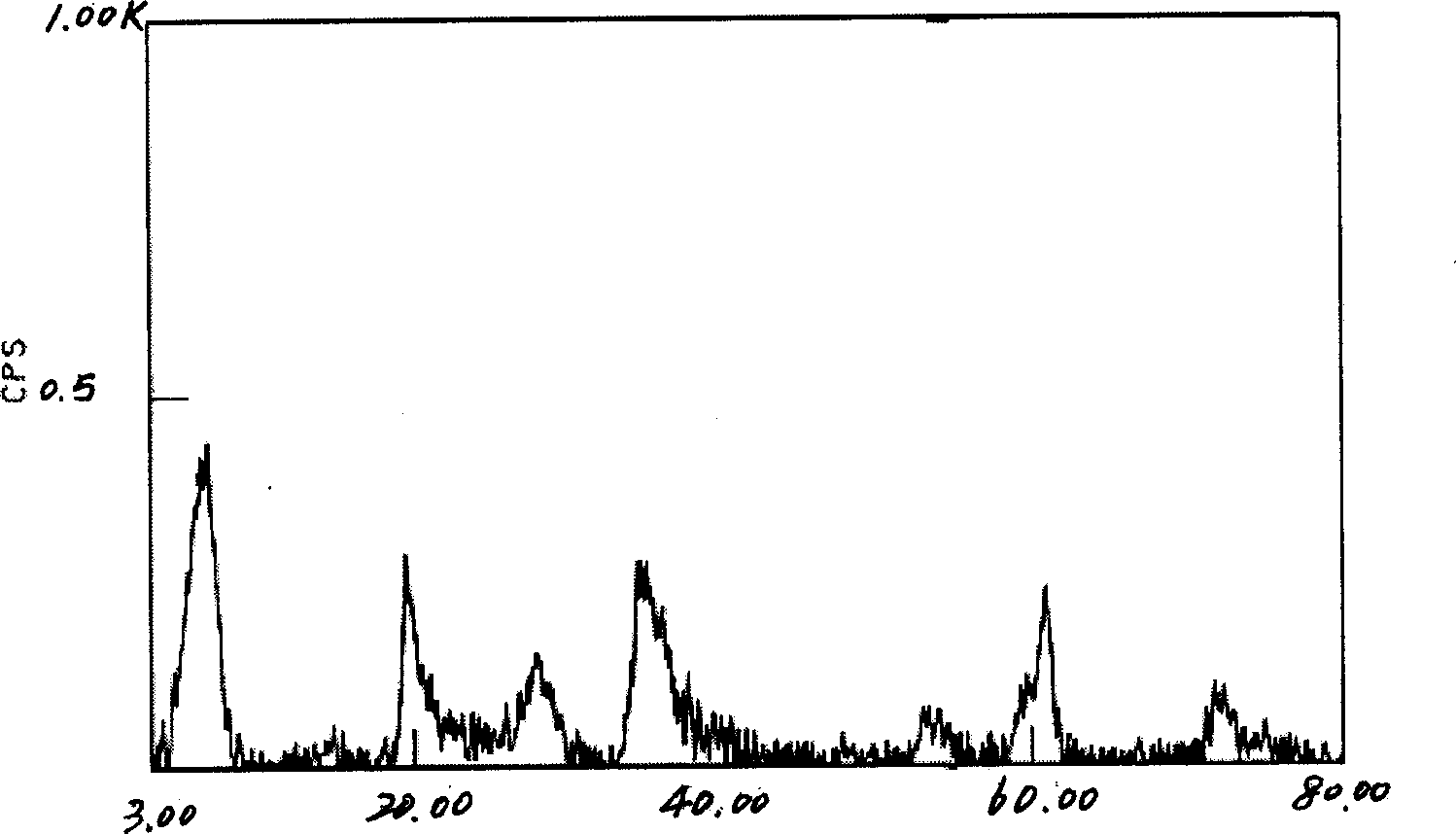
[0029] Get 1000g concentrated brine, its chemical composition weight percent is: LiCl 1.20%; NaCl 1.82%; KCl 0.28%; MgCl 2 28.60%; MgSO 4 3.25%; B 2 o 3 1.76%; H 2 O 63.09%; 1214.07g liquid potassium sodium silicate (aK 2 O·bNa 2 O·cSiO 2 ·nH 2 O, where K 2 O: 5.5%; Na 2 O: 5.5%; SiO 2 24.0%) was added into the concentrated brine, stirred to make it fully mixed, added NaOH solution to adjust the pH value of the mixed solution to 12, and used 9.18g LiCl 2 2H 2 O adjusts the ratio of the amount of magnesium, lithium and silicate in the mixed solution to be Mg 2+ : Li + : Si 4+ =2.7:0.3:4; then the mixed solution was placed in a high-pressure reactor, reacted at 200°C for 10 hours, cooled, and the gel-like product was taken out, washed by centrifugation, dried, and ground into a powder. The white powder sample obtained by XRD test is magnesium silicate hectorite clay. Its composition is: Na 2 O 2.96%; Li 2 O 1.18%; MgO 28.60%; SiO 2 62.98%; H 2 O 4.28%. Th...
Embodiment 3
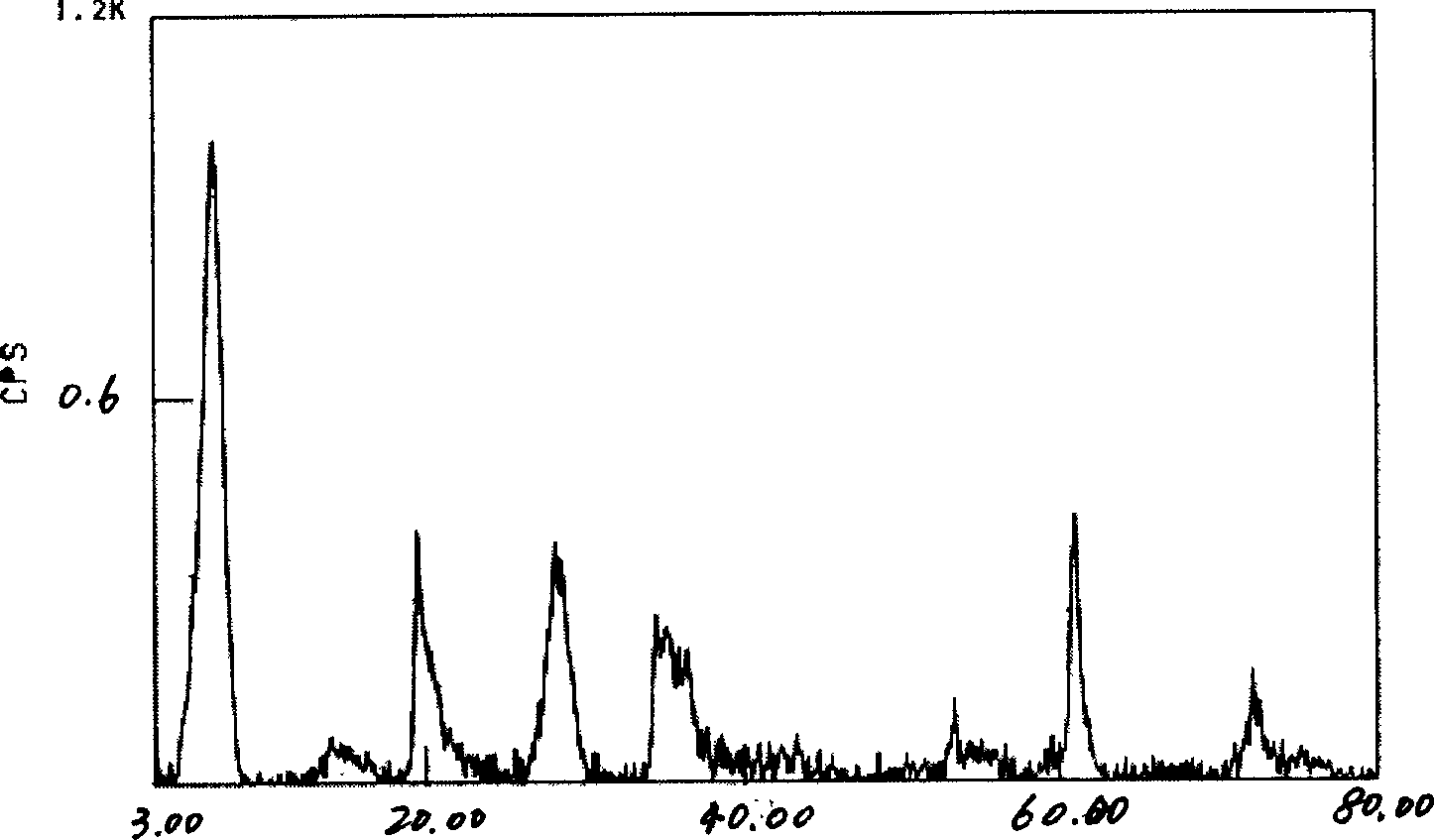
[0031] Get 1000g concentrated brine, its chemical composition weight percent is: LiCl 2.50%; NaCl 1.42%; KCl 0.18%; MgCl 2 29.13%; MgSO 4 3.75%; B 2 o 3 1.66%; H2 O 61.37%. 1630.10g liquid potassium silicate (K 2 SiO 3 ·nH 2 O) add in the concentrated brine, stir to make it fully mix, add NaOH solution to adjust the pH value of the mixed solution to 14, and use MgO to adjust the ratio of the amount of magnesium, lithium and silicate in the mixed solution to be Mg 2+ : Li + : Si 4+ =2.7:0.3:4; then the mixed solution was placed in a high-pressure reactor, reacted at 150° C. for 14 hours, cooled, and the gel product was taken out, washed by centrifugation, dried, and ground into a powder. The white powder sample obtained by XRD test is magnesium silicate hectorite clay. Its composition is: Na 2 O 1.03%; Li 2 O 1.16%; MgO 28.72%; SiO 2 63.07%; H 2 O 6.02%. The recovery rate was 89%.
[0032] Using concentrated salt lake brine as raw material, adjust the ratio o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com