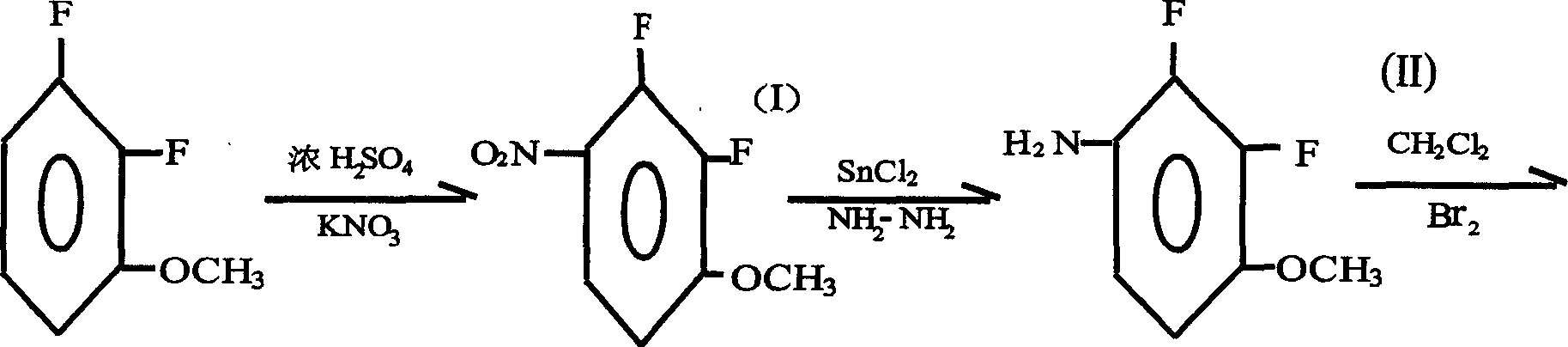
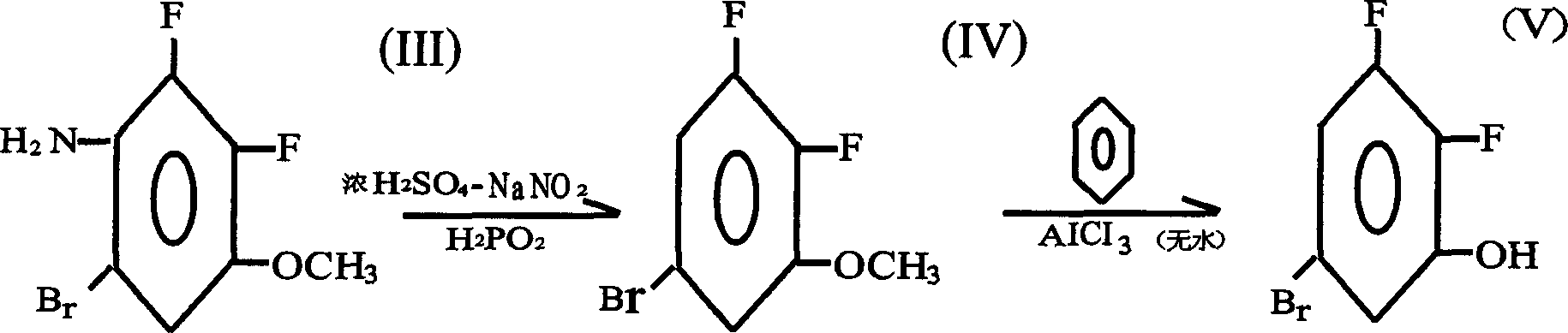
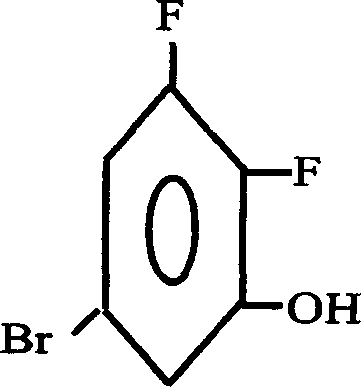
Preparation method of 2.3-difluoro-5-bromophenol
A technology of bromophenol and difluoroanisole is applied in the field of preparation of 2.3-difluoro-5-bromophenol, can solve problems such as low yield, inconsistent electronic effect, few products, etc., achieves high purity and is easy to purify Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 1. Nitration reaction: Synthesis of 2.3-difluoro-4-nitroanisole
[0021] (1) Ingredients and Mixing:
[0022] 2.3-Difluoroanisole: Concentrated sulfuric acid: Potassium nitrate 1:2:1~1.5(mol)
[0023] (2) Loading and operation
[0024] 1000ml three-necked glass reaction flask, equipped with mechanical stirring and thermometer, then add 144g of 2.3-difluoroanisole, 200g of concentrated sulfuric acid, 10g of cetyltrimethylammonium bromide, 75g of water, cool down in an ice-salt water bath below 10°C and stir , adding potassium nitrate kNO 3 106g, the total volume of the reaction mixture should not exceed 2 / 3 of the total volume of the reaction bottle. Stir for 8 hours below 10°C, track with the gas chromatograph, until no raw materials remain, put into ice water and suction filter to obtain a yellow-white solid, wash and dry .
[0025] (3) Purification treatment:
[0026] The dried solid was recrystallized from ethanol, and dried by suction filtration to obtain 180 g...
Embodiment 2
[0056] 1. Nitration reaction: Synthesis of 2.3-difluoro-4-nitroanisole
[0057] (1) Ingredients and Mixing
[0058] 2.3-Difluoroanisole: concentrated sulfuric acid: potassium nitrate = 1: 2: 1.05 (mol)
[0059] (2) Loading and operation
[0060] 500ml three-necked glass reaction flask, equipped with mechanical stirring and a thermometer, then add 72g of 2.3-difluoroanisole, 100g of concentrated sulfuric acid, 5g of cetyltrimethylammonium bromide, 38g of water, and stir in an ice-salt water bath to cool down below 10°C , adding potassium nitrate KNO 3 53g, after the addition, the total volume of the reaction mixture should not exceed 2 / 3 of the total volume of the reaction bottle. Stir for 8 hours below 10°C, track with the gas chromatograph, until no raw materials are left, put into ice water and suction filter to obtain a yellow-white solid. Wash and tumble dry.
[0061] (3) Purification treatment
[0062] The dried solid was recrystallized with ethanol, filtered and dri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com