Tumourolytic anticancer recombined adenovirus with tumour target direction and idioctonia
A technology of recombinant adenovirus and adenovirus, applied in antitumor drugs, virus/phage, drug combination, etc., can solve problems such as low infection rate, achieve enhanced infection, dose-expanding effect and anti-cancer ability, inhibit growth and spread Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1: Recombinant construction of oncolytic adenovirus.
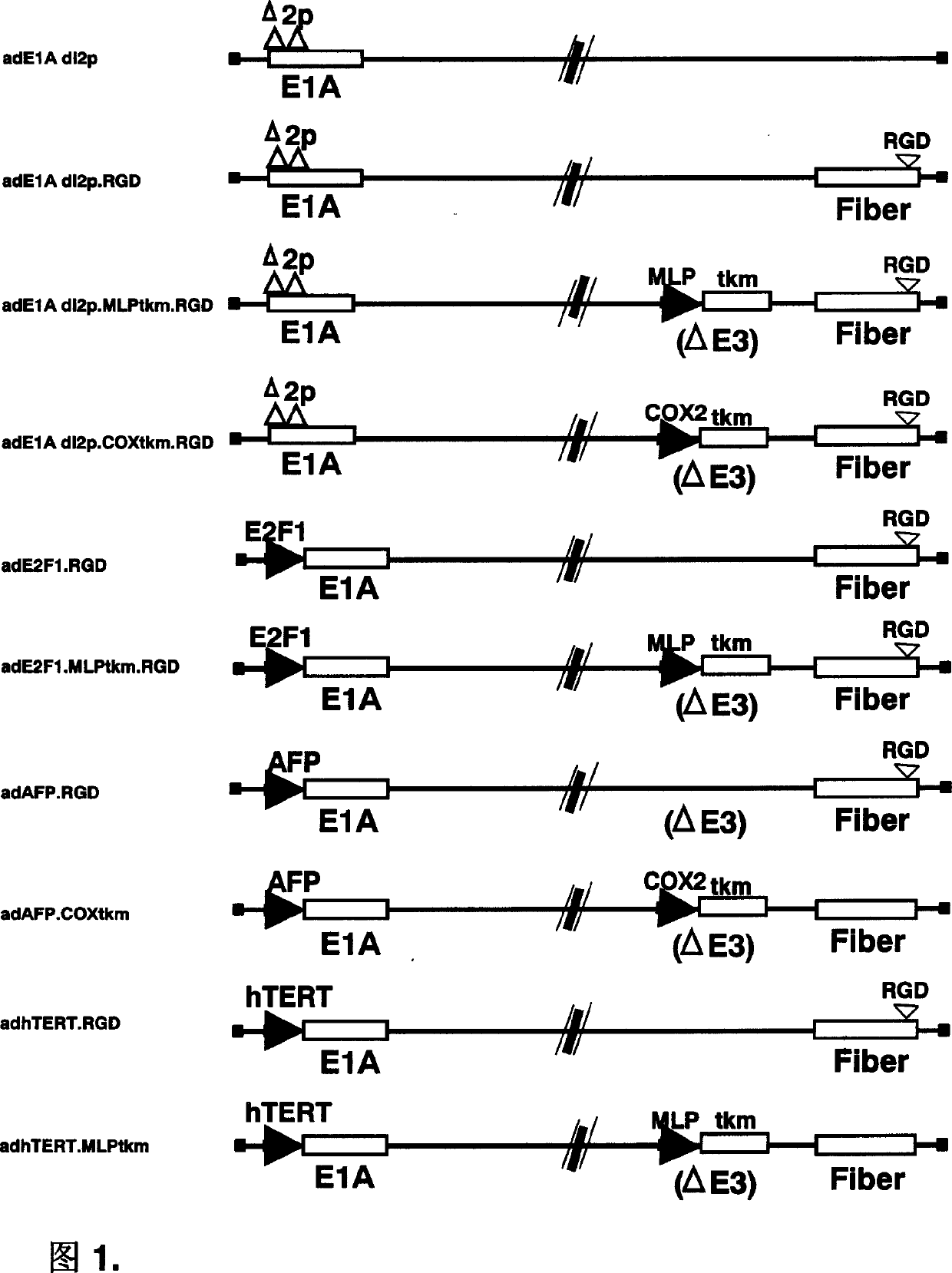
[0058] There are ten different oncolytic recombinant adenoviruses in the present invention. The specific production methods and steps of various viruses have been described in the previous technical solutions and will not be repeated here. Figure 1 is a schematic diagram of the genome DNA of these ten recombinant adenoviruses, mainly indicating the sites and genes of the genetic modification of ad5 in the present invention.
Embodiment 2
[0059] Example 2: Detection of self-replication ability of recombinant adenovirus by plaque method.
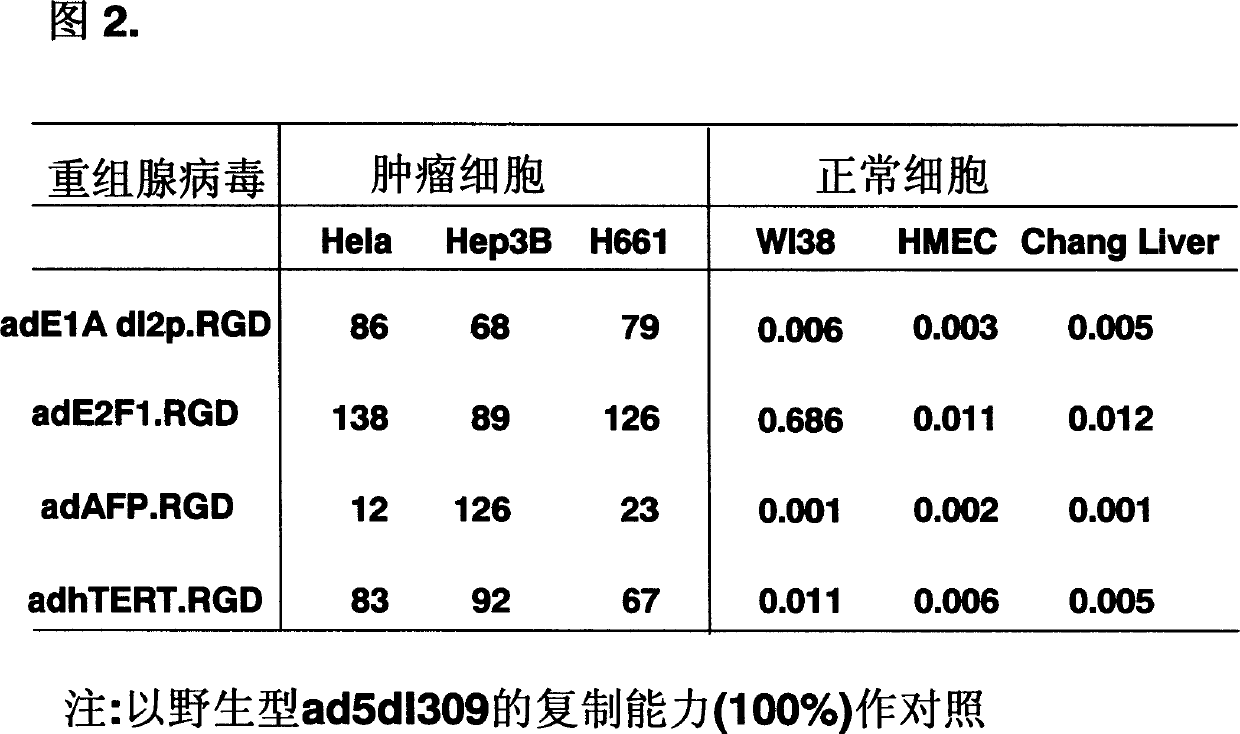
[0060] In order to evaluate the replication ability of the recombinant adenovirus of the present invention, we used wild-type ad5d1309 (its E3 has partial deletion and insertion mutation) as a control, and infected human liver cancer cell lines Hep3B, Hep3B, Cervical cancer cell line Hela, lung cancer cell line H661, normal liver cell Chang liver, normal breast epithelial cell HMEC and normal lung fibroblast WI38. After three days, cells were harvested. After three freezing and thawing at -80°C and 30°C to release the virions, the cell supernatant was used to infect HEK 293 cells to determine the viral load (pfu) by phagocytosis. In the same cell line, the comparison of the amount of virus produced by the recombinant virus and the wild-type virus can indicate the replication ability of the recombinant virus. Figure 2 lists the replication capabilities of representative recom...
Embodiment 3
[0061] Example 3: Detecting the effect of adding RGD short peptide to the fibrin terminal section on the infectivity of virus tumor cells.
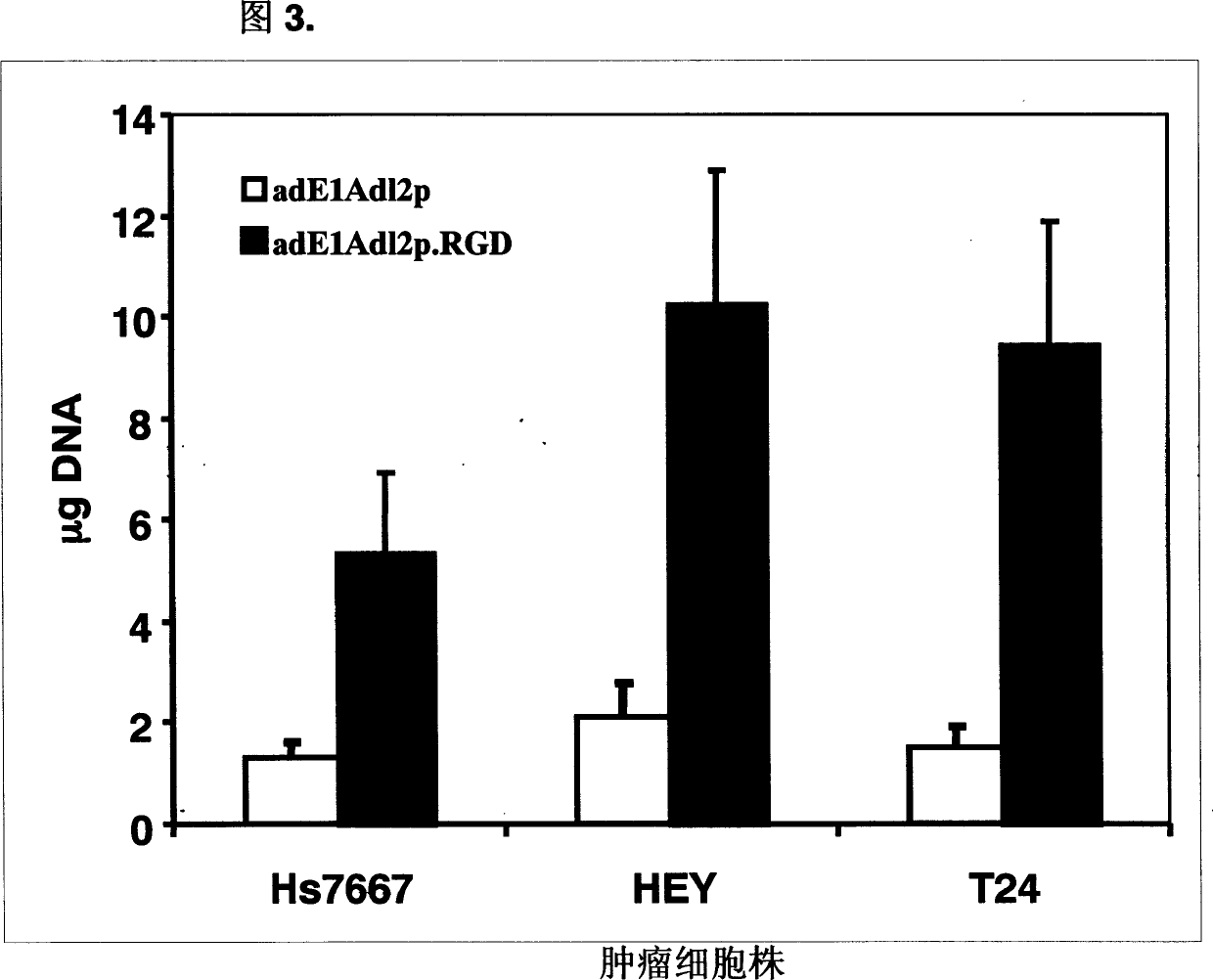
[0062] We selected human bladder cancer cell line T24, ovarian cancer cell line HEY and pancreatic cancer cell line Hs7667 to compare the infectivity of adE1Adl2p and adE1Adl2p.RGD. All three types of cancer cells have a moderate amount of adenovirus receptor CAR and a large amount of Cancer cells were infected with the same dose of the two viruses (MOI: 50). After 5 hours, the cells were harvested, and viral DNA was extracted from the cells. Then the 1Kb MLP fragment in the adenovirus DNA was amplified by quantitative PCR. The amount of PCR DNA obtained is shown in Figure 3. In these cancer cells, the infectivity of the recombinant adenovirus adE1Adl2p.RGD with RGD short peptide at the end of fibrin was 4-6 times that of the control virus adE1Adl2p. Obviously, the RGD short peptide can effectively enhance the recombinant adenovirus ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com