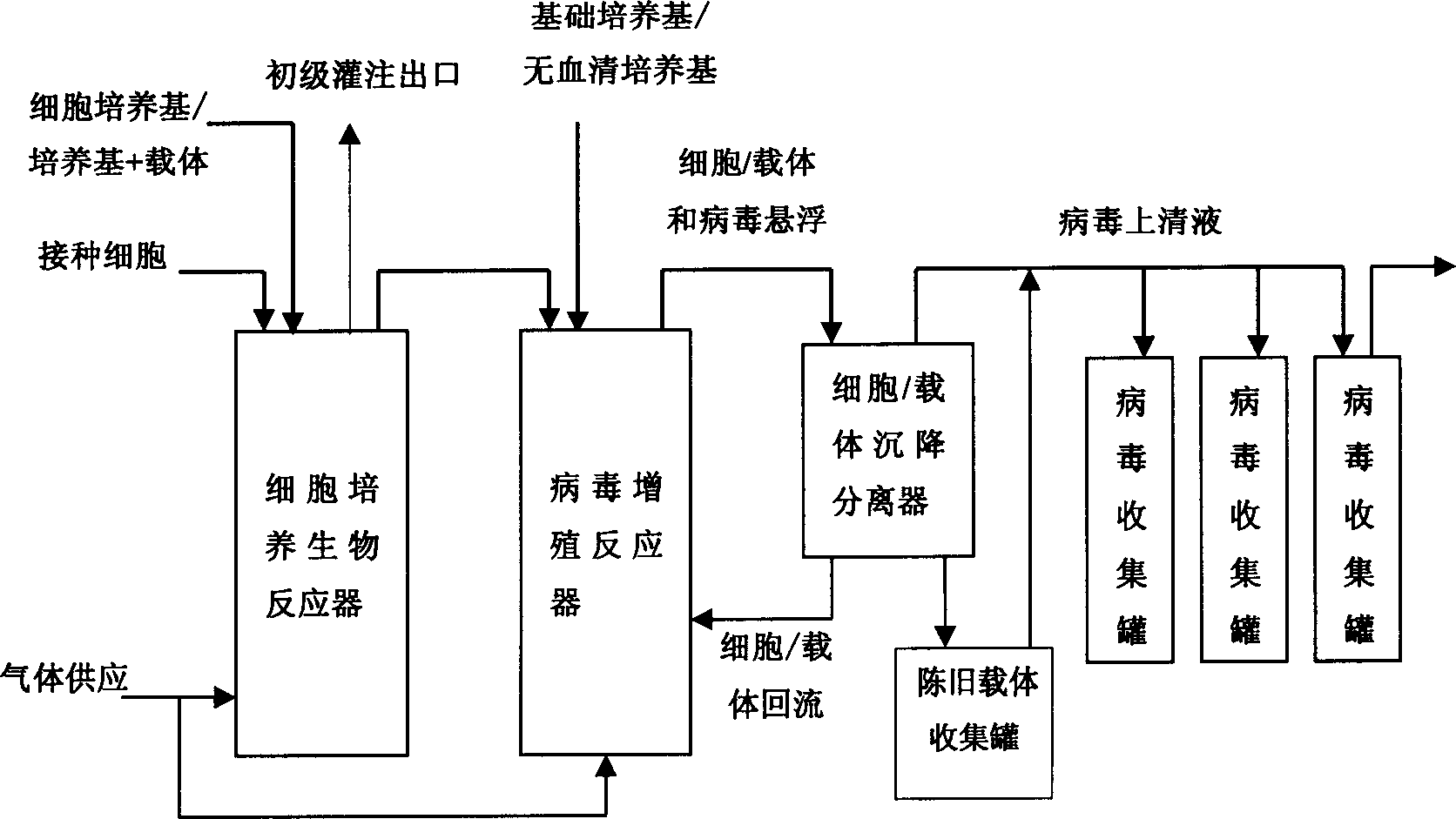
Method for safe continuous enclosed cell culture, virus production/ inactivation
A cell culture, closed technology, applied in the field of bioengineering, can solve problems such as unfavorable large-scale, continuous, safe production of virus vaccines, non-closed, etc., to achieve the effect of facilitating automatic control, improving operation flexibility, and reducing pollution opportunities.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0022] The above description generally illustrates the contents of the present invention, and the following examples will more directly reflect the situation of the present invention, but it should be pointed out that the examples listed here are only for the purpose of illustration, rather than limitation.
example 1
[0023] Example 1 Vero cell culture production / inactivation rabies virus
[0024] Medium: Medium 199 (Gibco), added with 5% fetal bovine serum, adjusted to pH7.0-pH7.2 with sodium bicarbonate.
[0025] Microcarriers and their treatment: Cytodex-1 (Pharmacia) is used as microcarriers, and the matrix is cross-linked dextran. When in use, dry microcarriers are added to a container siliconized with dimethyldichlorosilane, and the microcarriers are heated at room temperature with PBS solution. Fully soak, discard the supernatant, wash twice with PBS solution, replace with new PBS, sterilize at 121°C for 30 minutes, aspirate the supernatant, wash once with Medium 199 containing 5% fetal bovine serum, replace with new culture Base, equilibrate overnight in the incubator.
[0026] 1) Primary culture of cells
[0027] In the self-made siliconized 500mL stirred bioreactor (Spinner Flasks), the treated microcarriers were added at 3g / L, and Vero cells were inserted, and the inoculation...
example 2
[0036] Example 2 MDCK cell culture production / inactivation H3N2 influenza virus
[0037] This example was carried out with reference to the same steps as Example 1, and MDCK cells were used to culture H3N2 influenza virus.
[0038] Medium: Axcevir-MDCK serum-free medium (Excele Biotechnologies, France) was adjusted to pH7.0-pH7.2 with sodium bicarbonate
[0039] Microcarrier: Cytodex-3, dosage 5g / L.
[0040] Cell culture process: seeding density 1.5×10 5 cells / mL, microcarrier Cytodex-3, dosage 5g / L, culture bioreactor is self-made 500mL stirred bioreactor (Spinner Flasks) with siliconization treatment, cultured at 37℃, dissolved oxygen 40%, stirring speed 50rpm, primary The rate was 600mL / day, and after 2 days of inoculation, the cell density was 4.67×10 6 About cells / mL, continuous culture was started, the sampling rate was 300mL / day, samples were taken and counted regularly within 5 days, and the cell density was maintained at 4.21×10 6 cells / mL, it was found that the c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com