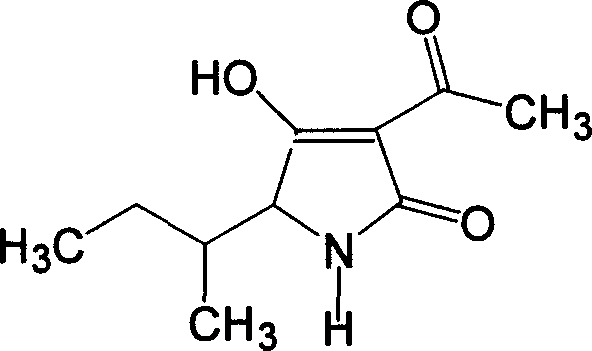
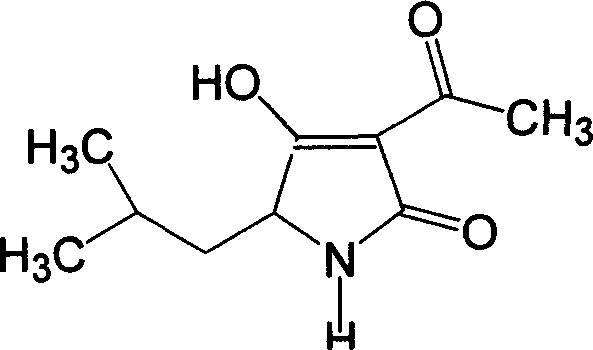
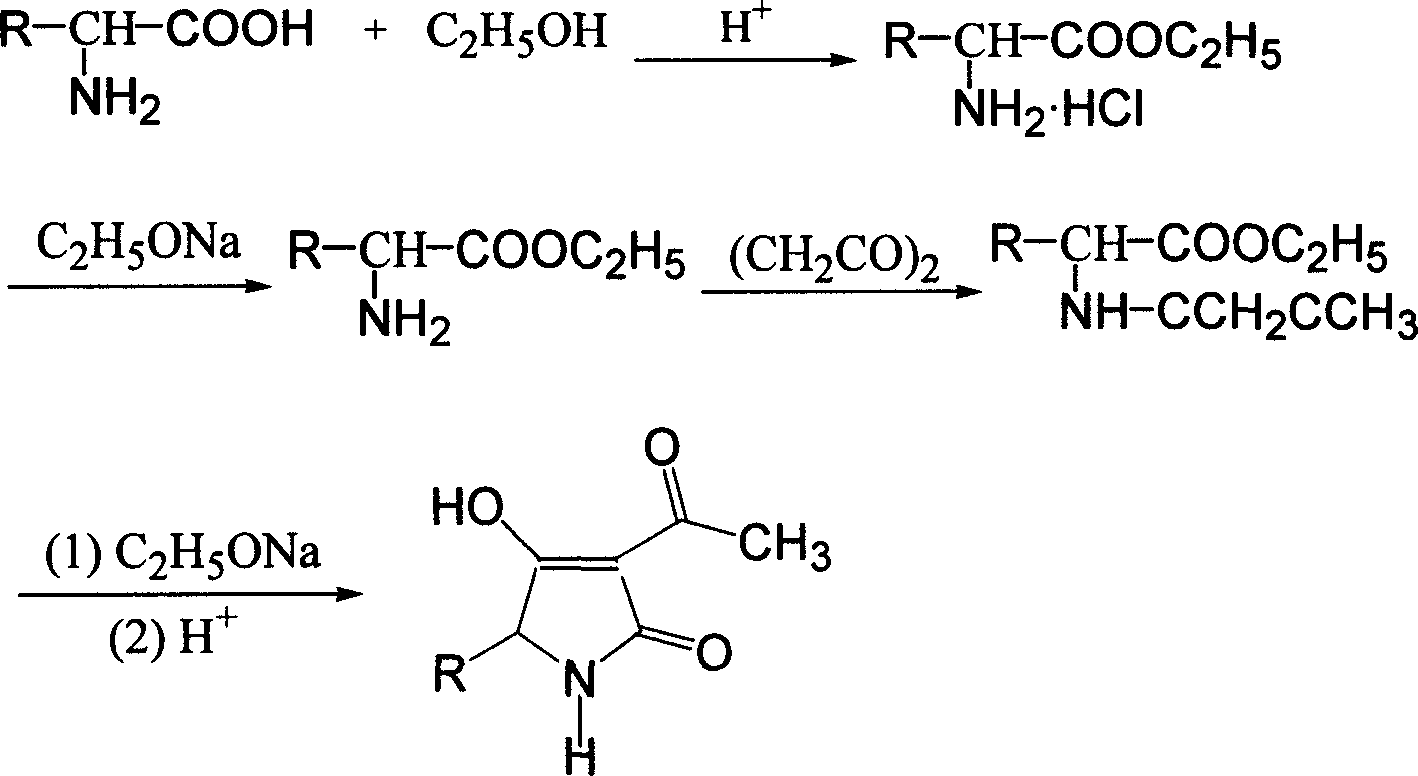
Synthesis of tenuazonic acid and iso-tenuazonic acid
A technology of alternaria tenoic acid and amino acid, which is applied in the direction of organic chemistry, can solve the problems of unsuitability for industrial production, poor safety, cumbersome purification, etc., and achieve simple production equipment requirements, low production cost, and easy operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Add 30mL of absolute ethanol to a dried 100mL three-neck flask equipped with a thermometer and a stirrer, add 0.8g (0.022mol) of dry HCl gas, add 2.63g (0.02mol) of isoleucine, heat, reflux, Stirring, reaction 3h, left overnight. Add 1.5g (0.022mol) of sodium ethoxide (newly made), stir and react for 0.5h. 1.85 g (0.022 mol) of diketene was added dropwise, and the drop was completed within 1 h. During the dropwise addition, the temperature was controlled below 10° C., and then stirred and reacted at room temperature for 4 h. Add 20 mL of benzene, 1.56 g (0.023 mol) of sodium ethoxide (newly produced), reflux, stir, react for 3 hours, let stand overnight, remove the solvent under reduced pressure, dissolve with 30 mL of water, extract with ethyl acetate 2×10 mL, and use 10 % of dilute sulfuric acid to neutralize, and then extracted with ethyl acetate 3×10mL, dried over anhydrous sodium sulfate, and evaporated to remove the solvent to obtain 3.6g of yellow viscous liquid...
Embodiment 2
[0029] Add 30mL of absolute ethanol to a dried 100mL three-necked flask equipped with a thermometer and a stirrer, add 0.8g (0.022mol) of dry HCl gas, add 2.63g (0.02mol) of leucine, heat, reflux, and stir , reacted for 3h and left overnight. Add 1.5g (0.022mol) of sodium ethoxide (newly made), stir and react for 0.5h. 1.85 g (0.022 mol) of diketene was added dropwise, and the drop was completed within 1 h. During the dropwise addition, the temperature was controlled below 10° C., and then stirred and reacted at room temperature for 4 h. Add 20 mL of benzene, 1.56 g (0.023 mol) of sodium ethoxide (newly produced), reflux, stir, react for 3 hours, let stand overnight, remove the solvent under reduced pressure, dissolve with 30 mL of water, extract with ethyl acetate 2×10 mL, and use 10 % of dilute sulfuric acid to neutralize, then extracted with ethyl acetate 3×10mL, dried over anhydrous sodium sulfate, distilled off 20mL of solvent, cooled and crystallized to obtain 3.2g of l...
Embodiment 3
[0031] Add 30L of absolute ethanol to the dried 100L reaction kettle equipped with a thermometer and agitator, feed 0.8kg (22mol) of dry HCl gas, add 2.63kg (20mol) of isoleucine, heat, reflux, and stir. Reaction 3h, left overnight. Add 1.5kg (22mol) of sodium ethylate (newly made), stir, and react for 0.5h. 1.85 kg (22 mol) of diketene was added dropwise, and the drop was completed within 1 hour. During the dropwise addition, the temperature was controlled below 10° C., and then stirred and reacted at room temperature for 4 hours. Add 20L of benzene, 1.56kg (23mol) of sodium ethoxide (newly made), reflux, stir, react for 3h, stand overnight, remove the solvent under reduced pressure, dissolve with 30L of water, extract with ethyl acetate 2×10L, and use 10% Neutralize with dilute sulfuric acid, then extract with ethyl acetate 3×10L, dry over anhydrous sodium sulfate, evaporate the solvent to obtain 3.56kg of yellow viscous liquid. After testing, the content of alternarinic a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com