Prawn white-spots syndrom virus (WSSV) membrane protein and its antibody
A vitiligo syndrome, virus membrane technology, applied in anti-viral immunoglobulins, antibodies, viral peptides, etc., can solve problems such as inability to completely solve problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1 viral membrane protein VP68, VP281, the expression of VP466 in Escherichia coli:
[0019] 1. Amplification of viral membrane protein VP68, VP281, VP466 cDNA
[0020] The cDNA sequences encoding viral membrane proteins VP68, VP281, and VP466 are amplified with PCR oligonucleotide primers corresponding to the 5' and 3' ends of the open reading frame of the DNA to obtain viral membrane proteins VP68, VP281, and VP466. DNA insert.
[0021] 2. DNA recombination of viral membrane proteins VP68, VP281, VP466
[0022] The amplified DNA fragments of VP68, VP281 and VP466 were respectively connected to the plasmid vector pGEX4T-2 by using genetic engineering tool enzymes. Then the E. coli DH-5a competent cells were transformed with the ligation mixture, and spread on LB plates (containing Amp 100 μg / ml). Colonies were picked, plasmids were extracted, and the VP68, VP281, and VP466 DNA fragments identified by PCR, enzyme digestion, and sequencing were correctly ins...
Embodiment 2
[0026] Example 2 Antibody Preparation
[0027]The purified recombinant viral membrane proteins VP68, VP281, and VP466 obtained in Example 1 were respectively emulsified with an equal volume of complete Freund's adjuvant, and the mice were subcutaneously injected with 50-100 μg / 0.2 ml of the emulsified protein. Ten days later, the same antigen emulsified with incomplete Freund's adjuvant was re-injected for booster immunization to immunize animals to produce antibodies. Booster immunization was carried out at least 3 times every 10 days. The titer and specificity of the obtained antisera were analyzed.
[0028] After reading the above teaching content of the present invention, those skilled in the art can make various changes and modifications to the present invention, and these equivalent forms also fall within the scope defined by the appended claims of the present application.
Embodiment 3
[0029] Embodiment 3 antiviral experiment
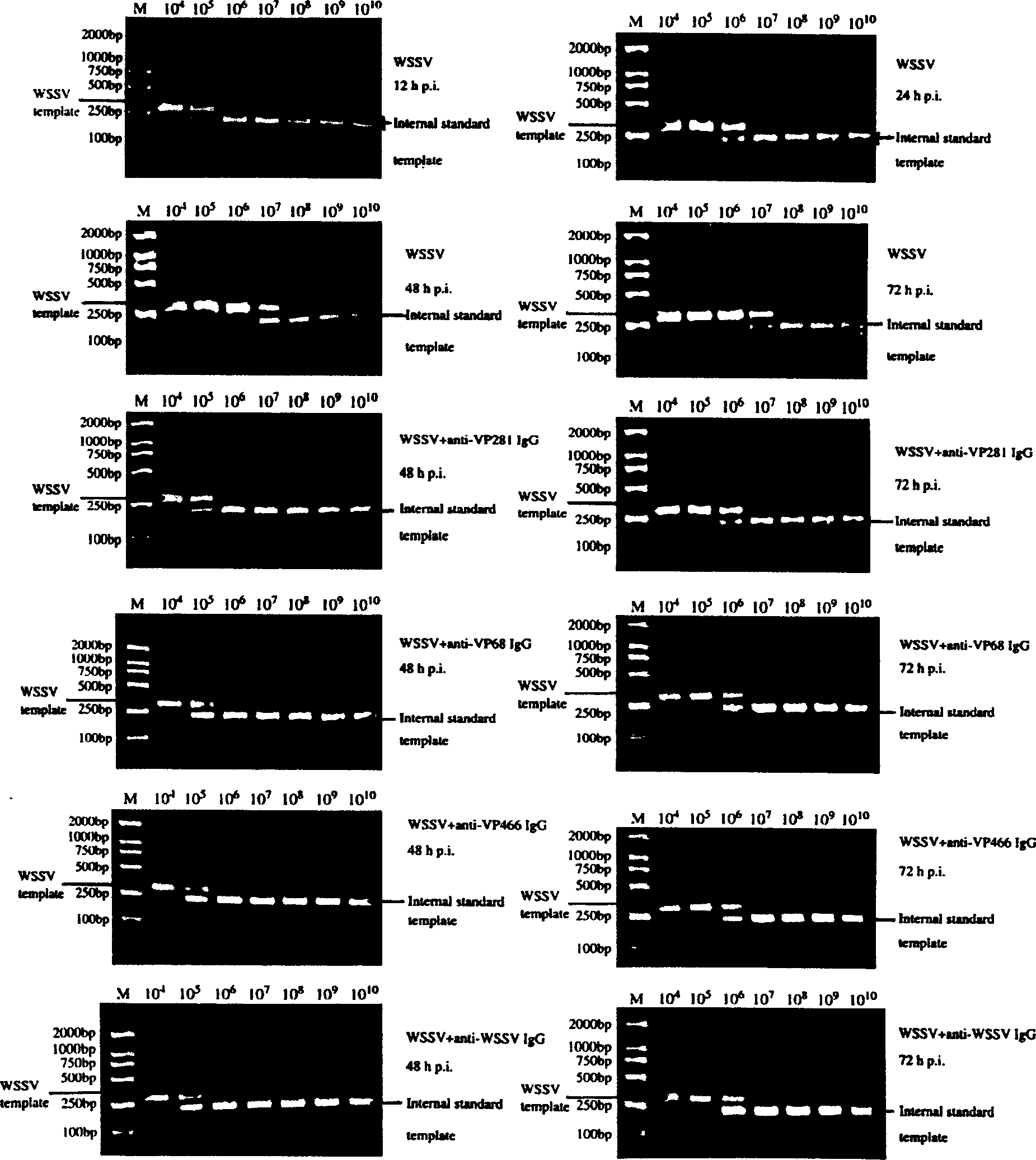
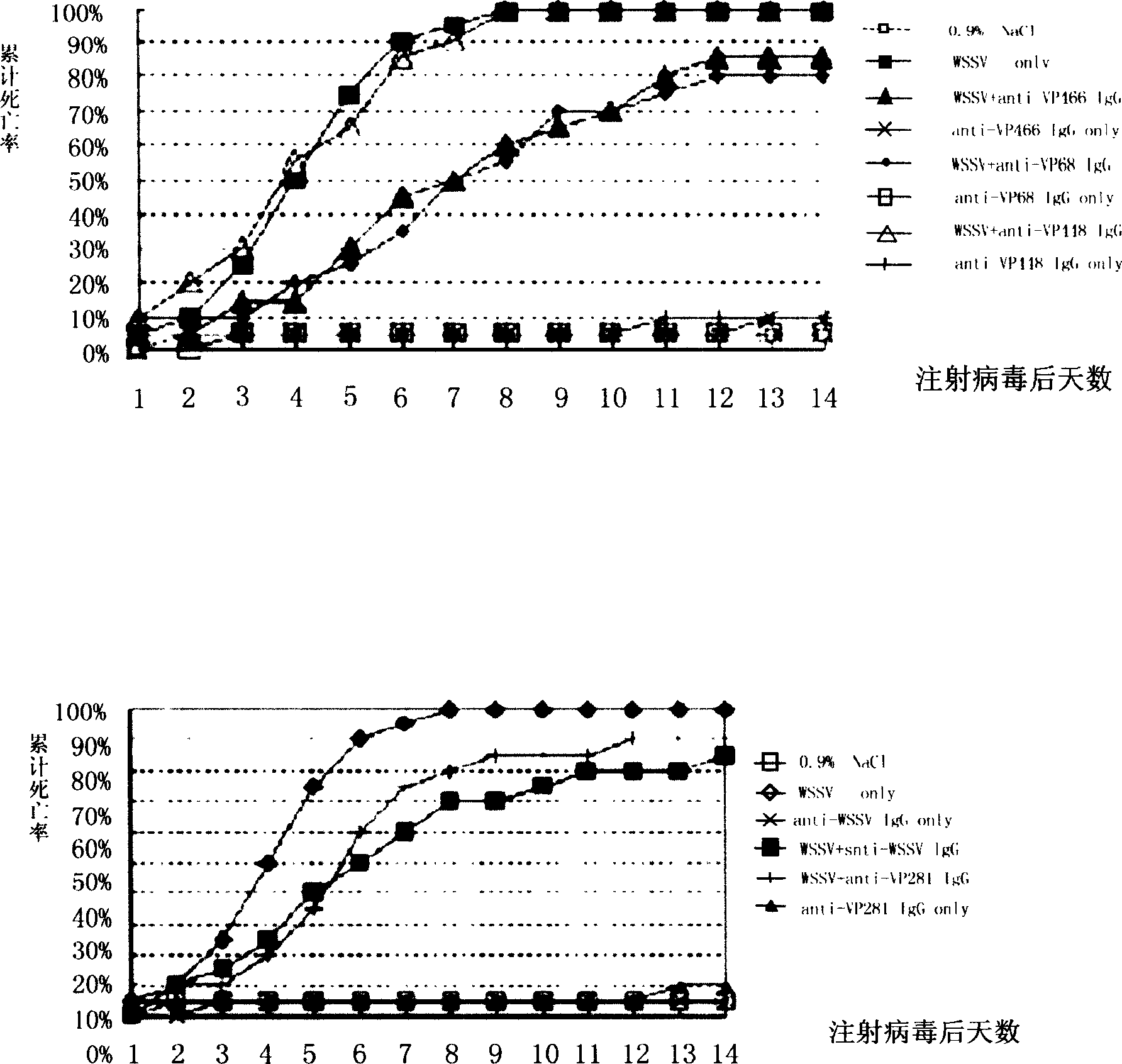
[0030] The polyclonal antibody obtained in Example 2 was mixed with the prawn white spot syndrome virus liquid respectively, left at room temperature for 1 hour, and then injected into 20 experimental prawns through the subcutaneous muscle, and each prawn was injected with 106 virus particles, Observed for 14 days, counted the cumulative mortality of the prawns within 14 days, compared with the prawns injected only with saline or only injected with prawn white spot syndrome virus, and analyzed the antiviral function of VP68, VP281, VP466 polyclonal antibodies. The experimental results are shown in accompanying drawings 1 and 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com