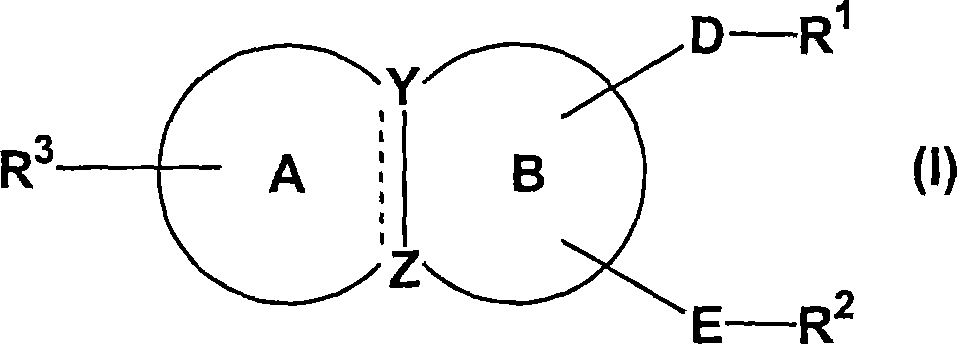
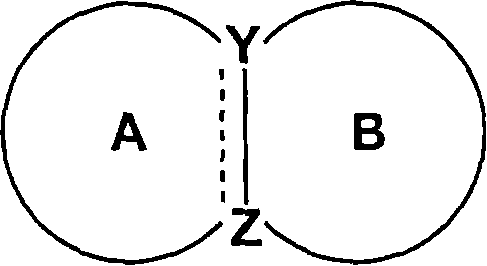
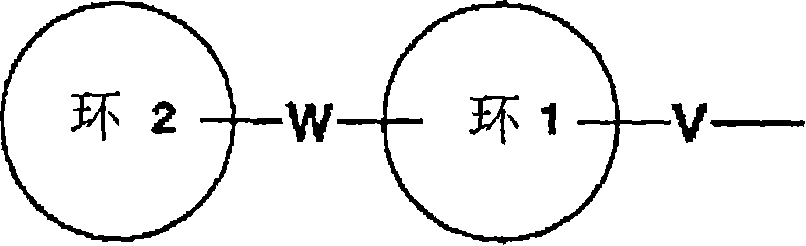
Condensed ring compound and use thereof
A technology of compounds and cyclic groups, which is applied in the field of fused ring compounds and their applications, and can solve problems such as the unclarified function and role of cysLT2 receptors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0559] The invention is illustrated by the following non-limiting examples and biological examples.
[0560] Solvents in parentheses in chromatographic separation and TLC represent eluents or developing solvents, and the ratios of solvents used in chromatographic separation and TLC are volume ratios. NMR indicates 1 Solvents in parentheses in H-NMR and NMR represent solvents used in the measurement. TFA stands for trifluoroacetic acid.
[0561] The nomenclature of the present invention is according to ACD / Name (trade name; Advanced Chemistry Development Inc.), which generates IUPAC rule nomenclature.
Embodiment 1
[0562] Example 1: 2-(phenylmethyloxy)-3-nitrobenzoic acid
[0563] Add benzyl bromide (50.0 mL) and potassium carbonate (66.3 g) to a solution of 2-hydroxy-3-nitrobenzoic acid (36.6 g) in N, N-dimethylformamide (500 mL), and the mixture is heated at 60° C. After stirring overnight, the reaction mixture was poured into water, the resulting mixture was extracted with a mixture of ethyl acetate and n-hexane (1:1), the organic layer was dried with water, saturated brine, anhydrous sodium sulfate and concentrated, and the residue was dissolved in In a mixture of tetrahydrofuran (100 mL) and methanol (200 mL), the resulting mixture was stirred at 50°C for 30 minutes, the reaction mixture was concentrated, the residue was acidified with 2N hydrochloric acid, extracted with ethyl acetate, the organic layer was washed with water and saturated brine, and anhydrous After drying over sodium sulfate and concentrating, the residue was recrystallized from isopropanol (50 mL) / n-hexane (200 mL...
Embodiment 2
[0565]Example 2: tert-butyl (2-(phenylmethyloxy)-3-nitrophenyl)carbamate
[0566] At room temperature, diphenylphosphoryl azide (24.9 mL) was added dropwise to a solution of the compound (30.0 g) prepared in Example 1 and triethylamine (16.2 mL) in toluene (440 mL), and the reaction mixture was stirred at 80° C. for 2 hours, tert-butanol (52.6 mL) was added to the reaction mixture, the mixture was stirred at 80°C for 3 hours, the reaction mixture was cooled to room temperature, washed successively with water, 0.1N hydrochloric acid, water, saturated aqueous sodium bicarbonate and saturated brine, After drying over sodium sulfate and concentration, the obtained residue was purified by silica gel column chromatography (n-hexane:ethyl acetate=9:1) to obtain the title compound (32.98 g) having the following physical data.
[0567] TLC: Rf 0.40 (n-hexane:ethyl acetate=9:1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com