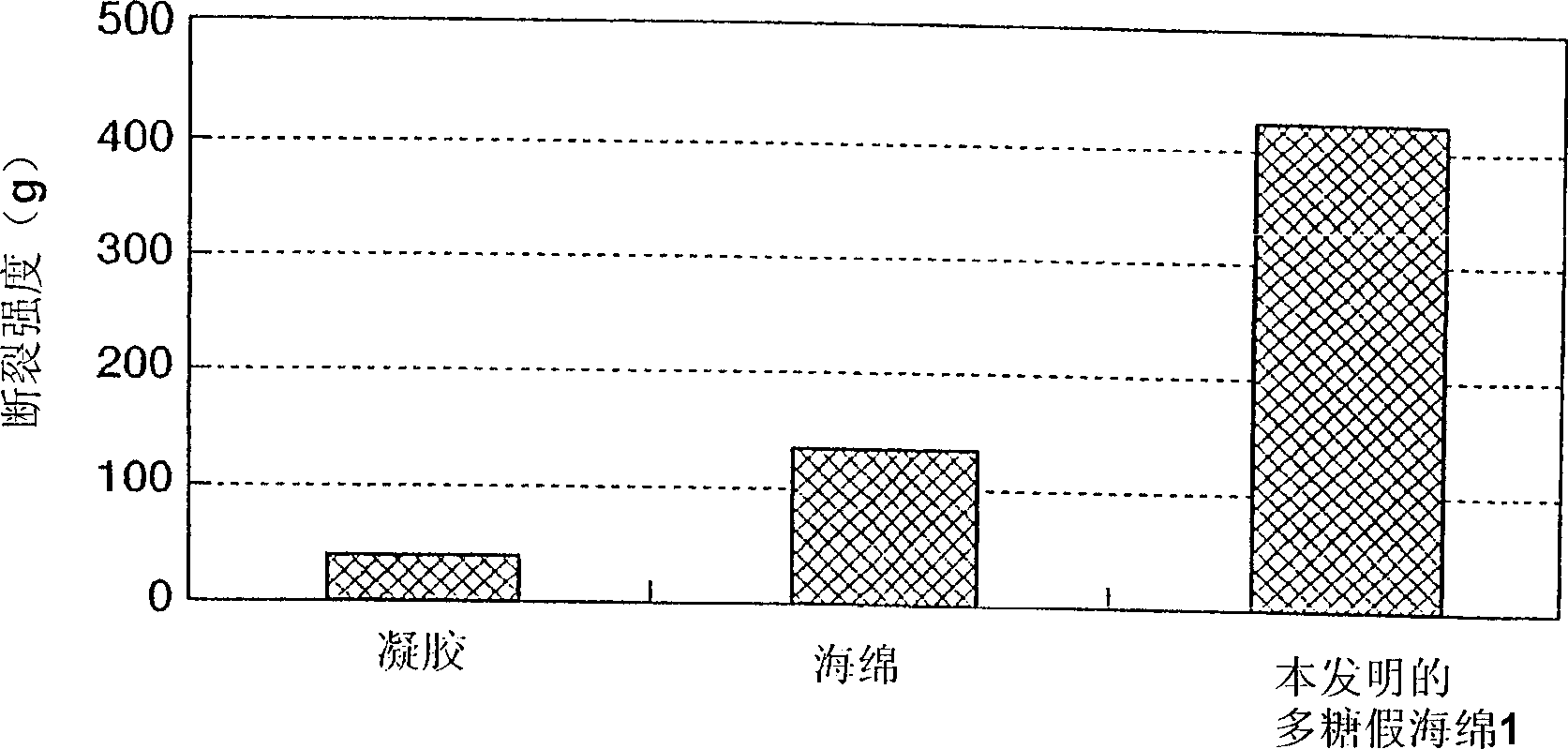
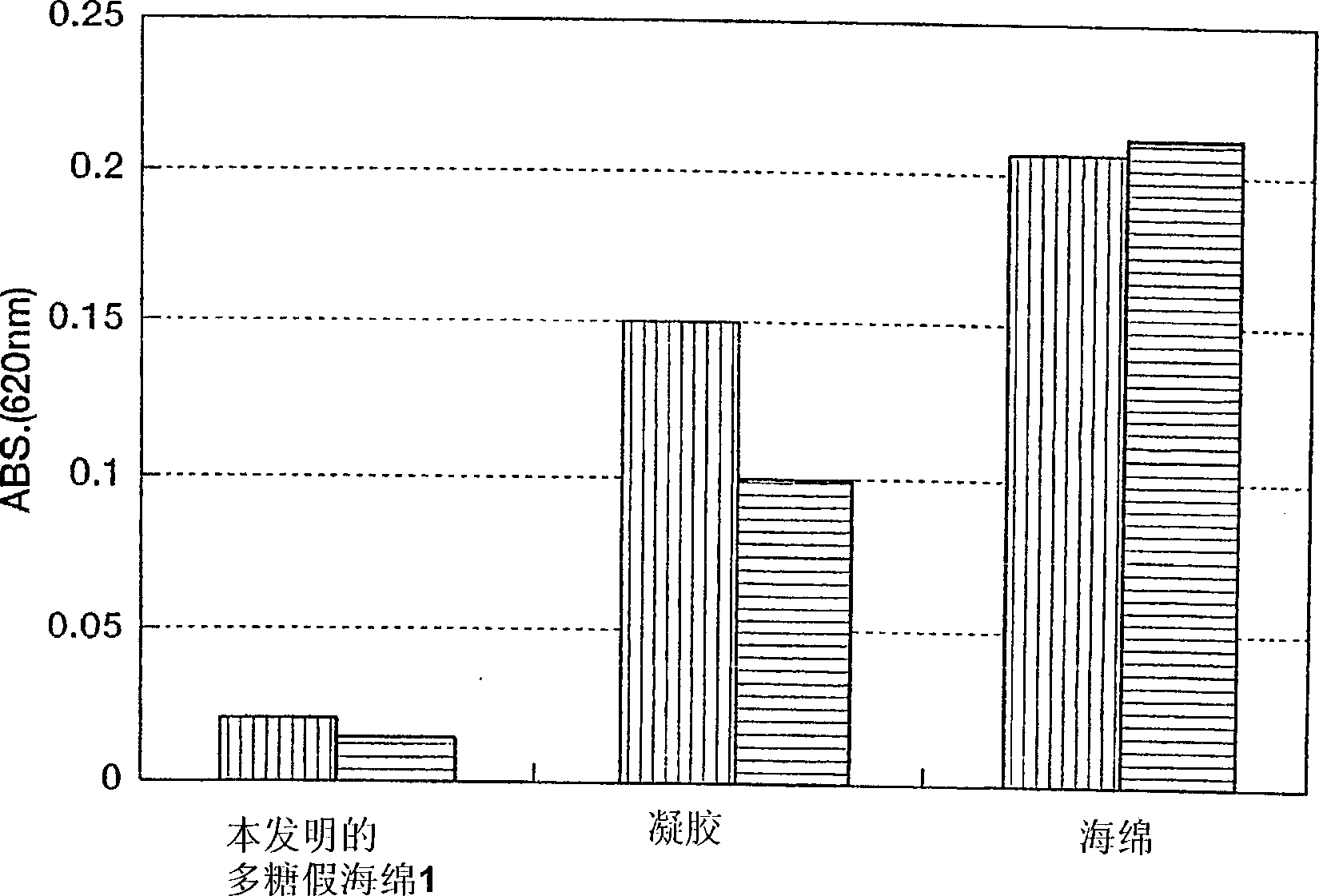
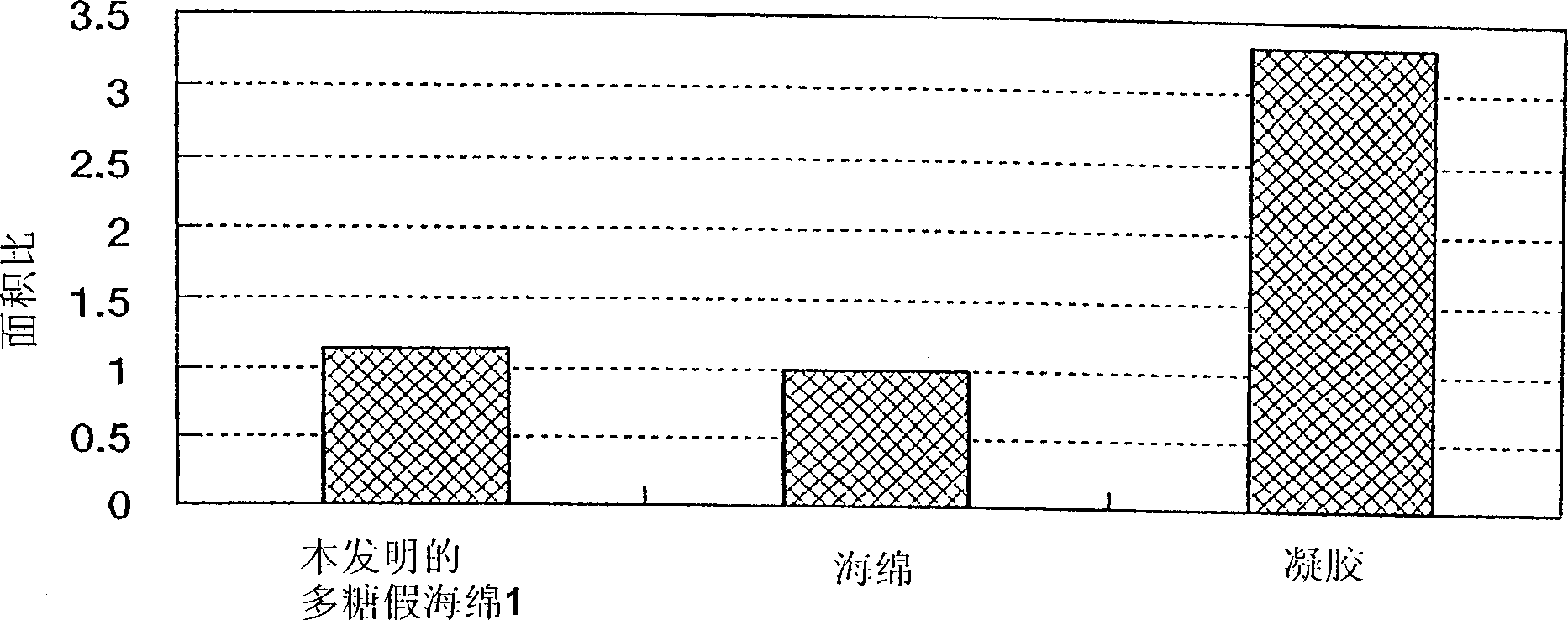
Polysaccharide pseudo-sponge
A polysaccharide and sponge technology, applied in the field of polysaccharide pseudosponge, can solve problems such as easy cracking of gel sheets, and achieve the effects of high barrier effect, high strength and excellent biodegradability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0096] Hereinafter, the present invention is described in more detail through examples, but the examples are only for illustration and are not intended to limit the scope of the present invention. Meanwhile, technical terms and measurement methods used in the Examples are defined as follows.
[0097] (1) Degree of substitution of photoreactive groups
[0098] In the case of introducing a photoreactive group to the carboxyl group of a glycosaminoglycan as a polysaccharide, the degree of substitution of the photoreactive group refers to the number of photoreactive groups introduced per disaccharide repeating unit. percentage. The amount of glycosaminoglycan required to calculate the degree of substitution of photoreactive groups was measured by the carbazole method using its calibration curve. When using cinnamic acid or substituted cinnamic acid as the photoreactive substance, the amount of cinnamic acid was measured by absorbing The measurement method (measurement wavelength...
manufacture Embodiment 1
[0101] Production Example 1 (production of photoreactive hyaluronic acid):
[0102] A mixed solution containing 250 mL of water and 375 mL of dioxane was added to 500 g of 1 wt% sodium hyaluronate (comb-derived product, manufactured by Seikagaku Co., Ltd.; weight-average molecular weight: 900,000) under stirring. in aqueous solution. The resulting mixed solution was mixed successively with the following solutions at room temperature and stirred for 2.5 hours: a solution prepared by dissolving 860 mg of N-hydroxysuccinimide in 2 mL of water (0.6 equivalents / hyaluronic acid disaccharide unit (mol / mol) ), a solution prepared by dissolving 717mg of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDCl / HCl) in 2mL of water (0.3 equivalents / hyaluronic acid disaccharide unit (mol / mol)) and 903mg aminopropyl cinnamate hydrochloride (HCl·H 2 N(CH 2 ) 3 A solution prepared by dissolving OCO-CH=CH-Ph, wherein Ph represents phenyl) in 2 mL of water (0.3 equivalents / disacch...
manufacture Embodiment 2
[0103] Manufacturing example 2 (manufacture of cross-linked hyaluronic acid gel):
[0104] (1) The cinnamic acid-introduced hyaluronic acid obtained in Production Example 1 was dissolved in water for injection to prepare a 4 wt% aqueous solution thereof. The resulting solution was poured into a tempered glass plate mold with a cavity of 6cm x 2.5cm x 1mm (thickness), and then irradiated with ultraviolet light on each surface for 15 minutes under water-cooling conditions using a 3kW metal halide lamp to obtain a transparent Sheet gel (water content: 96wt%). The cross-linking ratio of the obtained gel was determined to be 30%.
[0105] (2) The same procedure as defined in (1) above was carried out, except that cinnamic acid-introduced hyaluronic acid having a degree of substitution of 4.6% was produced using the same method as defined in Production Example 1, thereby obtaining a transparent sheet-like gel . The cross-linking ratio of the resulting gel was determined to be 13....
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com