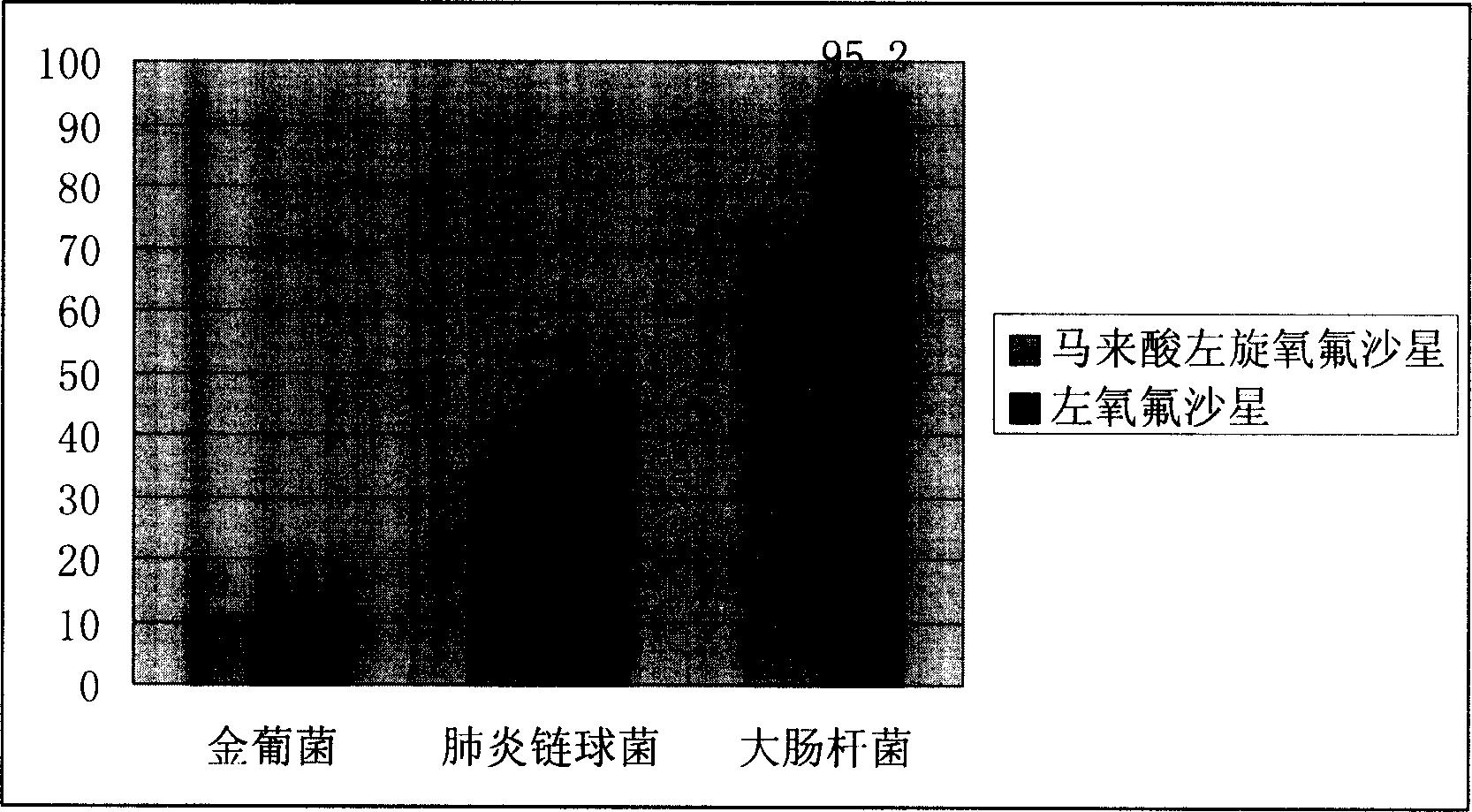
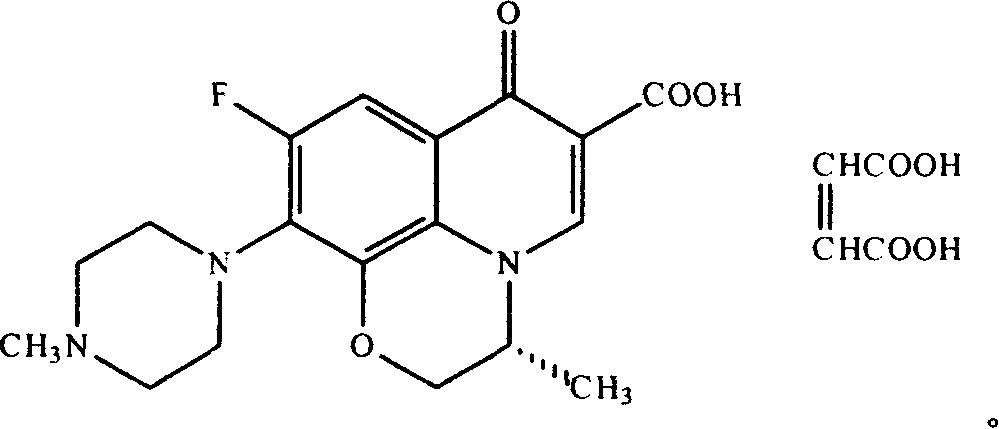
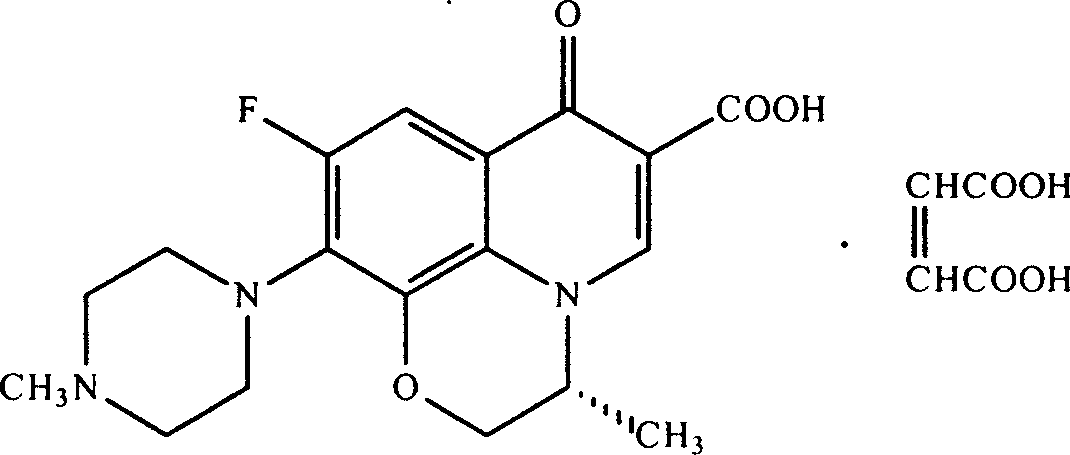
Levo ofloxacin derivative, its production and use
A technology of levofloxacin and derivatives, applied in the fields of levofloxacin derivatives, preparation and use thereof, can solve the symptoms of venous irritation or central nervous system reactions, adverse reactions and safety are not determined, serum trough Alanine aminotransferase increase and other problems, to achieve the effect of high crystallization rate, high safety, and complete salt formation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Synthesis of levofloxacin maleate crude product (levofloxacin and maleic acid molar ratio is 1: 1) add 20g (0.054mol) levofloxacin to 150ml absolute ethanol, heat to dissolve, Form a yellow transparent solution, weigh 6.58g (0.057mol) of maleic acid and add it to the levofloxacin ethanol solution, stir, heat, and reflux for 30 to 50 minutes, suction filter while it is hot, and wash the filter cake with absolute ethanol , dried in vacuo at 60°C to obtain the crude product of levofloxacin maleate;
Embodiment 2
[0049] Levofloxacin maleate crude product synthesis (levofloxacin and maleic acid molar ratio is 1: 0.5) 20g (0.054mol) levofloxacin is added in 140ml absolute ethanol, heated to dissolve, Form a yellow transparent solution, weigh 3.29g (0.029mol) of maleic acid and add it to the levofloxacin ethanol solution, stir, heat, and reflux for 30-50 minutes, suction filter while it is hot, and wash the filter cake with absolute ethanol , dried in vacuo at 60°C to obtain the crude product of levofloxacin maleate;
Embodiment 3
[0051] Synthesis of crude levofloxacin maleate (the molar ratio of levofloxacin to maleic acid is 1:2)
[0052] 20g (0.054mol) levofloxacin is added in 150ml absolute ethanol, is heated to dissolving, forms yellow transparent solution, then takes by weighing 13.16g (0.114mol) maleic acid and adds in levofloxacin ethanol solution, Stir, heat, and reflux for 30 to 50 minutes, suction filter while hot, wash the filter cake with absolute ethanol, and vacuum-dry at 60°C to obtain the crude product of levofloxacin maleate;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com