Preparation process of entecavir
A technology of entecavir and compounds, which is applied in the field of drug preparation, can solve the problems of harsh reaction conditions, high price, and high cost, and achieve the effects of high reaction efficiency, low price, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
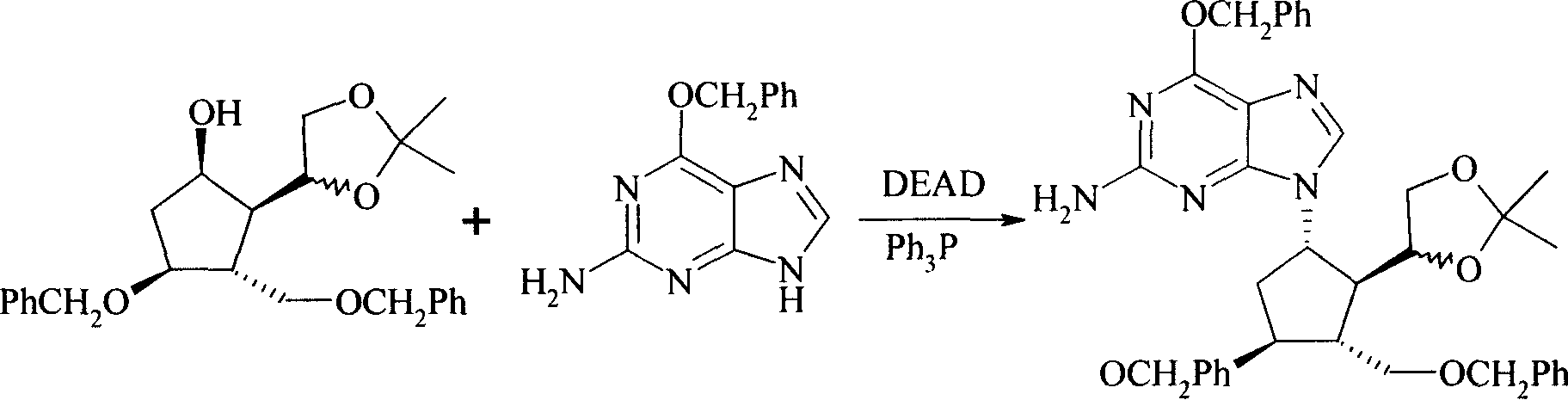
[0027] Example 1 (1S, 2R, 3R, 4S)-2-amino-6-benzyloxy-9-[4-benzyloxy-3-benzyloxymethyl-2-(2,2-dimethyl- [1,3]dioxolane)-cyclopentyl]-9H-purine (Formula IV)
[0028] (1R, 2R, 3R, 4S)-3-benzyloxymethyl-2-[2-(1-methoxy-1-methyl-ethoxy)-ethyl]-4-benzyloxy- Cyclopentanol (formula II) (41.2g, 0.1mol) and triphenylphosphine (12.6g, 0.05mol) were dissolved in anhydrous tetrahydrofuran (500ml), cooled to 0°C, and 2-amino-6-benzyloxy Ethyl-9H-purine (Formula III) (29.0 g, 0.12 mol). Keeping at 0°C, a solution of diethyl diazodicarboxylate (20.9 g, 0.12 mol) in tetrahydrofuran (200 ml) was added dropwise. After the dropwise addition, the mixture was stirred overnight at room temperature. The solvent was evaporated to dryness under reduced pressure to obtain formula IV (53 g), with a yield of 83%.
Embodiment 2
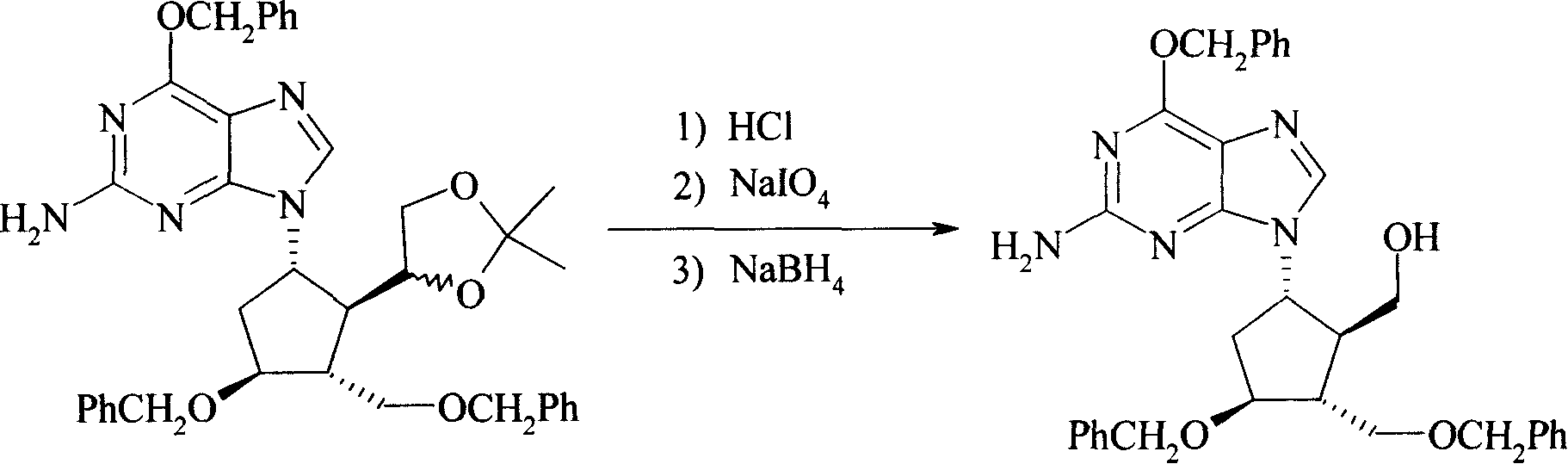
[0029] Example 2 (1S, 2R, 3R, 4S)-[5-(2-amino-6-benzyloxy-purine-9-generation-)-3-benzyloxy-2-benzyloxymethyl-cyclopentyl Base]-methanol (Formula V)
[0030] Concentrated hydrochloric acid (10 ml, 0.12 mol) was added dropwise to a solution of Formula IV (63.5 g, 0.1 mol) in methanol (500 ml), followed by stirring at room temperature for 3 hours. The solvent was distilled off under reduced pressure. Add methanol (500ml), cool to 0°C, add sodium periodate (25.7g, 0.12mol) in water (500ml), and stir at room temperature for 1 hour. Add sodium borohydride (7.6 g, 0.20 mol) and stir at room temperature for 1 hour. After filtration, the filtrate was concentrated, and then extracted with dichloromethane (300ml×3). The extracts were combined and dried over anhydrous sodium sulfate. After filtration, the filtrate was evaporated to dryness to obtain formula V (44g), with a yield of 78%.
Embodiment 3
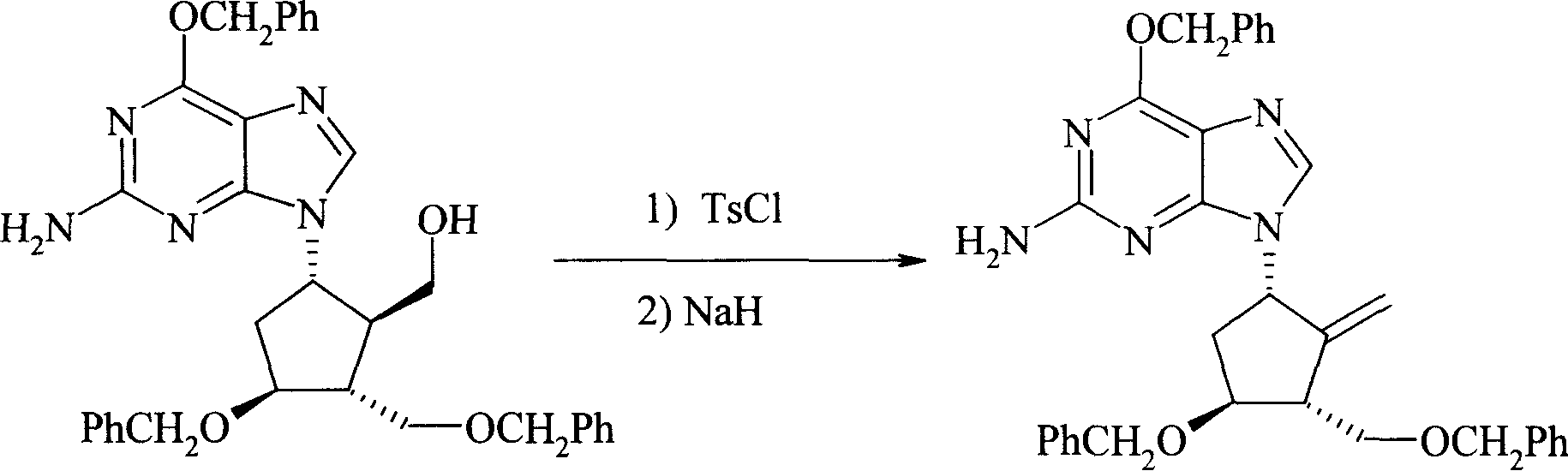
[0031] Example 3 (1S, 3R, 4S)-2-amino-6-benzyloxy-9-(4-benzyloxy-3-benzyloxymethyl-2-methylene-cyclopentyl)-9H- Purine (formula VI)
[0032] Add dichloromethane (500ml) to formula V (56.5g, 0.1mol), then add 4-dimethylaminopyridine (25g, 0.2mol), cool to 0°C, then add p-toluenesulfonyl chloride (23g, 0.12mol ), stirred at 0°C for 2 hours. Warm to room temperature and stir for 3 hours. Dichloromethane (500ml) was added for dilution, washed with water (500ml) and saturated sodium bicarbonate solution (500ml) respectively, and the organic layer was dried over anhydrous sodium sulfate. Filter and evaporate the filtrate to dryness. To the residue was added N,N-dimethylformamide (500ml).
[0033] 60% sodium hydride (12 g, 0.3 mol) was added to N,N-dimethylformamide (100 ml), then 2-methoxyethanol (40 ml, 0.5 mol) was added dropwise, and stirred for 1 hour. Cool to 0°C, slowly add the solution obtained above, and continue stirring at 0°C for 4 hours after the addition is complet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com