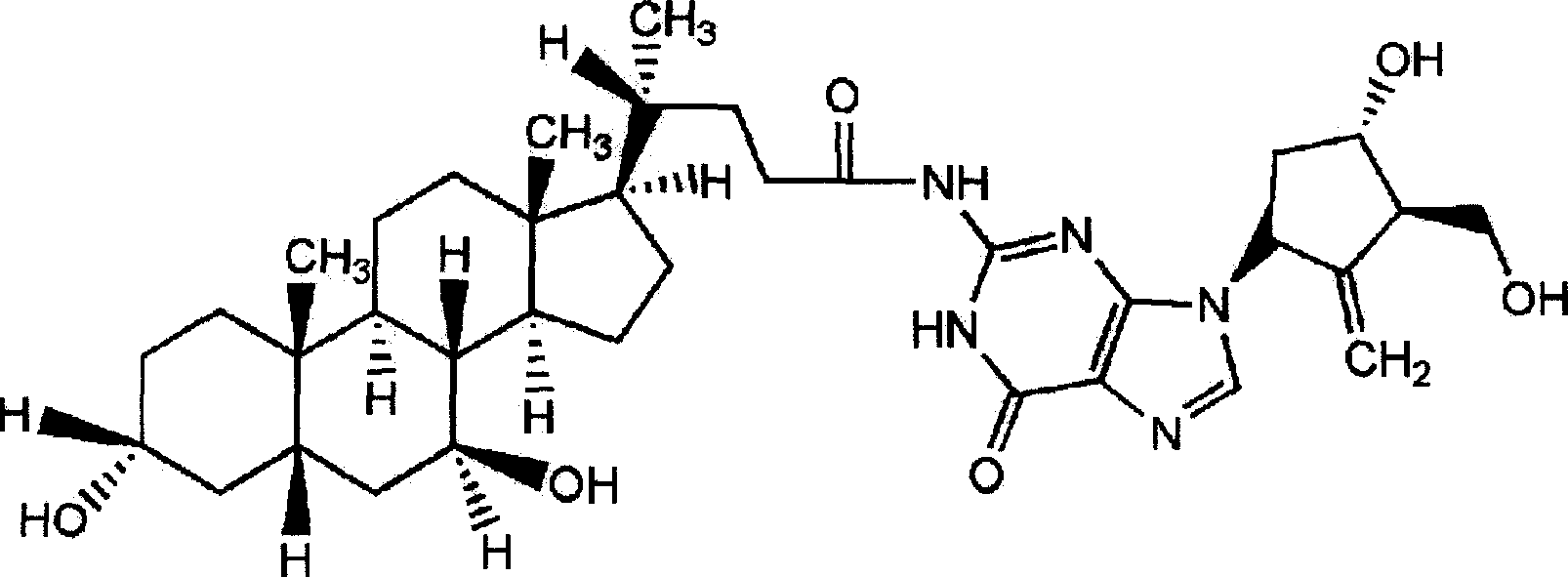
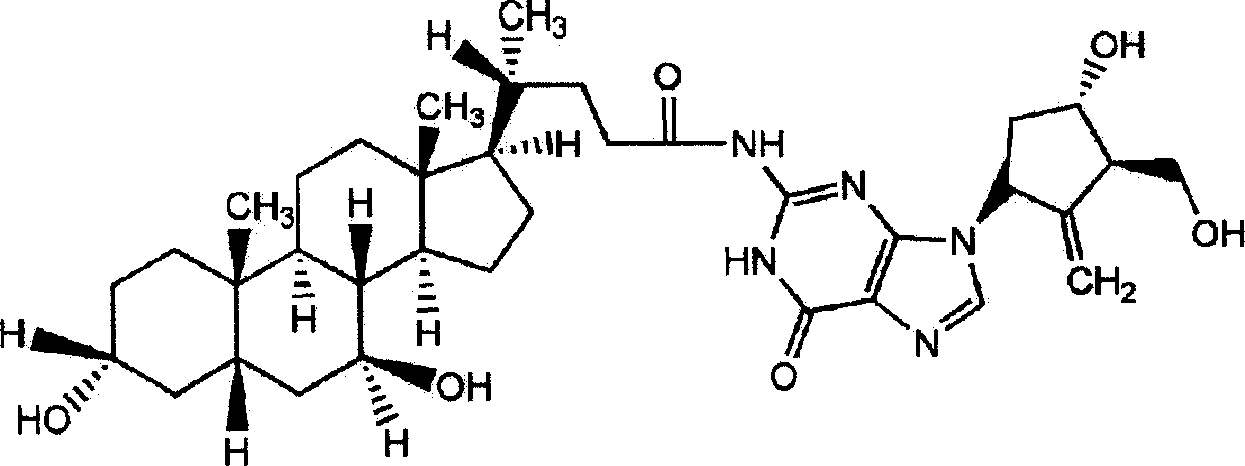
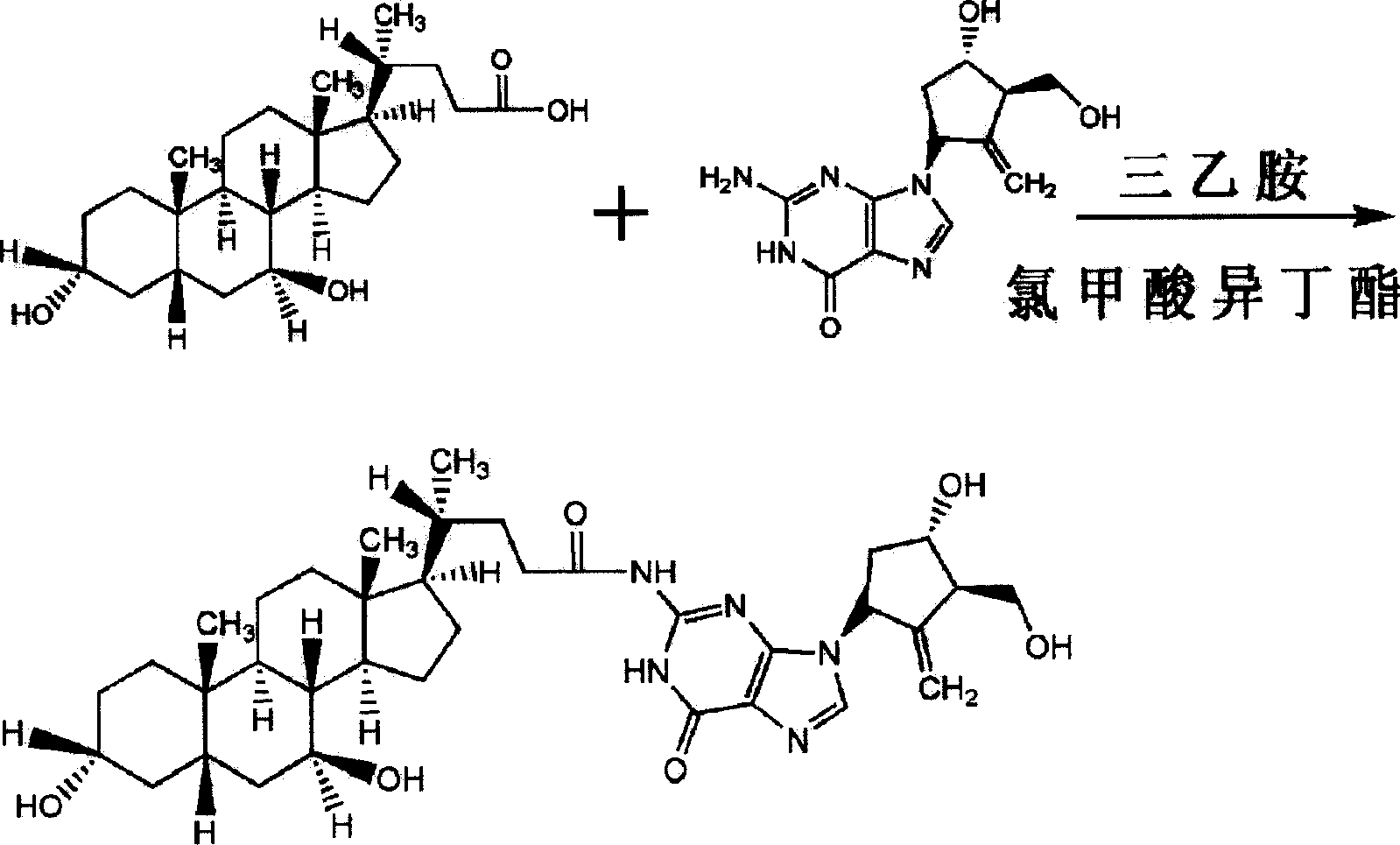
Ursodeoxycholic acid entecavir acidamide and preparation method and use thereof
A technology of ursodeoxycholic acid and oxycholic acid, which is applied in the field of treatment of hepatitis B virus infection, can solve the problems of large side effects and low curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: Preparation of ursodeoxycholic acid entecavir amide
[0029] Reaction formula:
[0030]
[0031] Operation steps Put 100mL DMF into the beaker, add (10mmol, 4.0g) ursodeoxycholic acid and (10mmol, 1.4ml) triethylamine under stirring, cool the temperature of the reaction solution to -15°C under stirring, then add ( 15mmol, 2mL) isobutyl chloroformate. After stirring for 2 minutes, a solution of entecavir (10 mmol, 2.8 g) and triethylamine (15 mmol, 2.2 mL) dissolved in 40 ml of DMF was added, and the temperature of the reaction solution was maintained at -15 ° C. After stirring the reaction mixture for 30 min, it was left at room temperature for 1 hour. Filter to remove triethylamine chloride therein, then distill and concentrate the filtrate under reduced pressure, add the concentrate to 400ml of ethanol and heat it appropriately to dissolve, filter and discard the insoluble matter, wash the filtrate twice with an appropriate amount of chloroform solut...
Embodiment 2
[0036] Embodiment 2: the preparation tablet of pharmaceutical composition:
[0037] Element
[0038] Preparation method: grind the raw materials and auxiliary materials separately, pass through a 100-mesh sieve, and set aside. Dissolve an appropriate amount of sodium carboxymethyl starch in water to obtain a 2% sodium carboxymethyl starch solution as a binder. Mix the main and auxiliary materials thoroughly. Use 2% sodium carboxymethyl starch solution as a binder to make a suitable soft material, pass through a 30-mesh sieve to granulate, dry the wet granules at about 60°C, pass through a 30-mesh sieve for granulation, add magnesium stearate and mix well . Determine the drug content in the granules, calculate the theoretical tablet weight, and compress the tablet. Take an appropriate amount of LE film coating agent, add it to 75% ethanol solution, stir to disperse it evenly, and obtain 7% LE film coating solution. Measure the tablet core content and dissolution ...
Embodiment 3
[0039] Embodiment 3: the preparation of pharmaceutical composition
[0040] Element
[0041] Preparation method: respectively pulverize the main drug and auxiliary materials in the prescribed amount through an 80-mesh sieve, and set aside. Get sodium carboxymethyl starch and add water to make 2% sodium carboxymethyl starch solution as a binder. The main ingredient and auxiliary materials are uniformly mixed in equal amounts, added with 2% sodium carboxymethyl starch solution, passed through a 20-mesh sieve to granulate, dried at 55°C, and granulated at 20-mesh to obtain dry granules. Add the dry particles to 100-mesh micronized silica gel and mix well. Put in capsules and get ready.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com