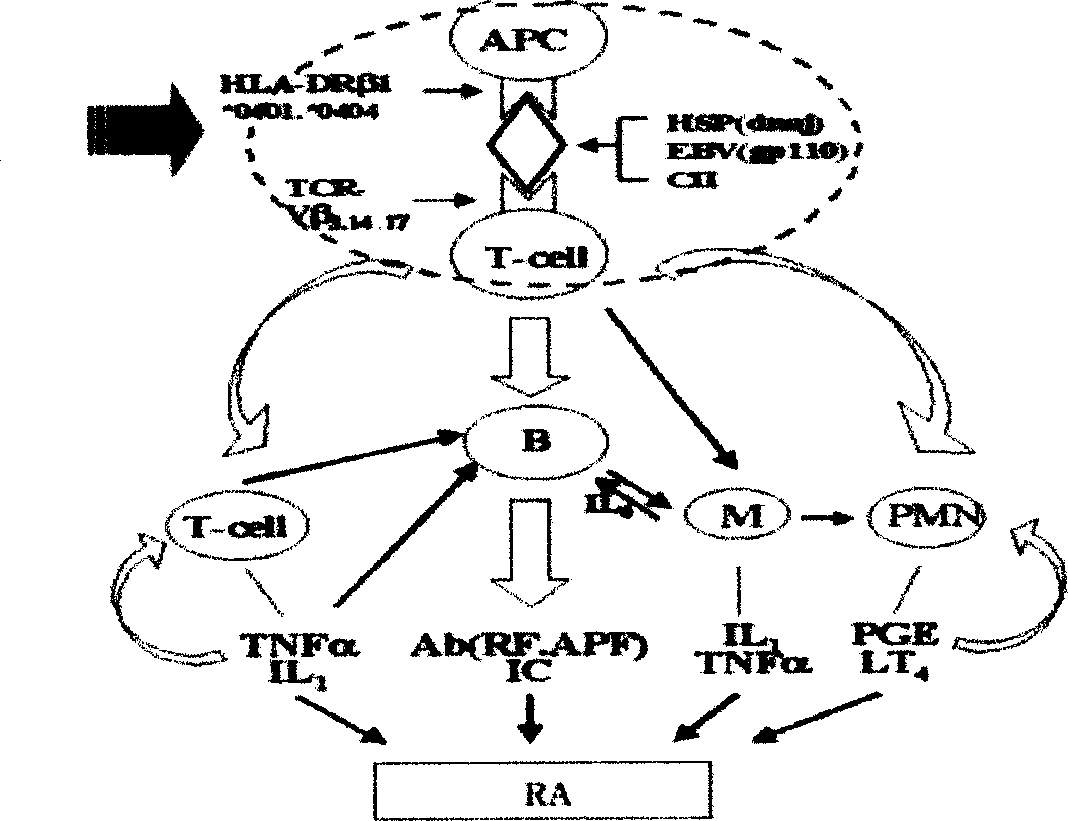
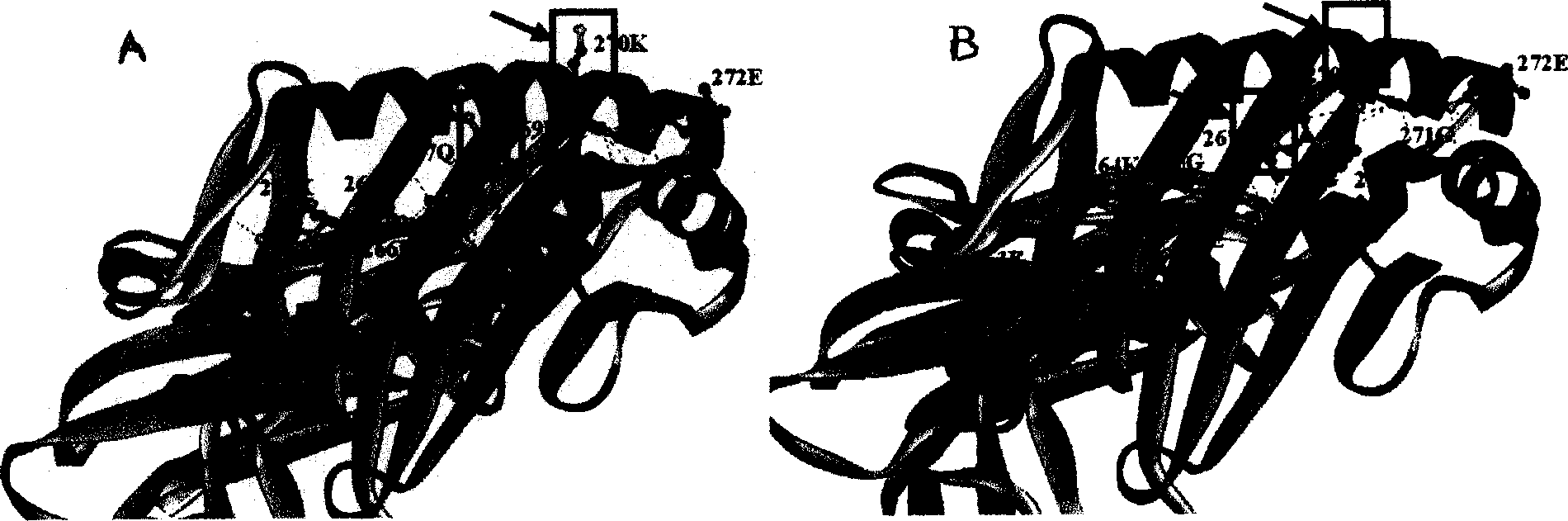Therapeutic effect of type II collagen allosteric peptide for treating rheumatoid arthritis
An allosteric peptide, rheumatoid technology, applied in medical preparations containing active ingredients, anti-inflammatory agents, peptide/protein components, etc., can solve problems such as many side effects, inability to inhibit rheumatoid arthritis, and inability of patients to take medication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: the synthesis of polypeptide
[0021] The amino acids (267Q and 270K) recognized by the TCR in the CII263-272 polypeptide were replaced with the A of the short side chain, thereby obtaining three CII263-272 allosteric peptides (altered peptide ligand, APL). See Table 1 for their sequences. Simultaneously, type II collagen 263-272 (FKGEQGPKGE) prototype peptide was synthesized. The above polypeptides were all synthesized by Shanghai Boya Company by solid-phase synthesis and purified by high-performance liquid chromatography. In order to facilitate the transport of the peptide into the cell, the amino terminus of the peptide is connected with myristic acid. The results of mass spectrometry showed that the sequence of the polypeptide was correct and the purity was over 95%.
[0022] Peptide name
CII263-272
APL1
APL2
APL3
Ma
---
---
---
F
---
---
---
...
Embodiment 2
[0025] Implementation 2: In silico simulation of the binding of CII263-272 prototype and allosteric peptides to HLA-DR4 molecules
[0026] Using the Red Hat Linux 9 operating system, using the HLA-DR4-CII1168-1179 crystal structure (PDB: 2SEB) as a template, the prototype of CII263-272 and allosteric peptides were tested on a CPU 2.4 GHz Xeon CPU×2 workstation and rheumatoid arthritis. Computer simulation experiment of HLA-DR4 molecular binding of the sensitive gene. The binding ability of each polypeptide to HLA-DR4 molecules was evaluated by SCORE scoring software. The size of the binding ability is expressed by pKd value (the negative logarithm of the dissociation constant Kd). The larger the pKd value, the stronger the intermolecular binding force.
[0027] The results show that the CII263-272 prototype peptide molecule can be well embedded in the antigen-binding groove of HLA-DR4, while the long side chains of 267Q and 270K protrude out of the antigen-binding groove, fo...
Embodiment 3
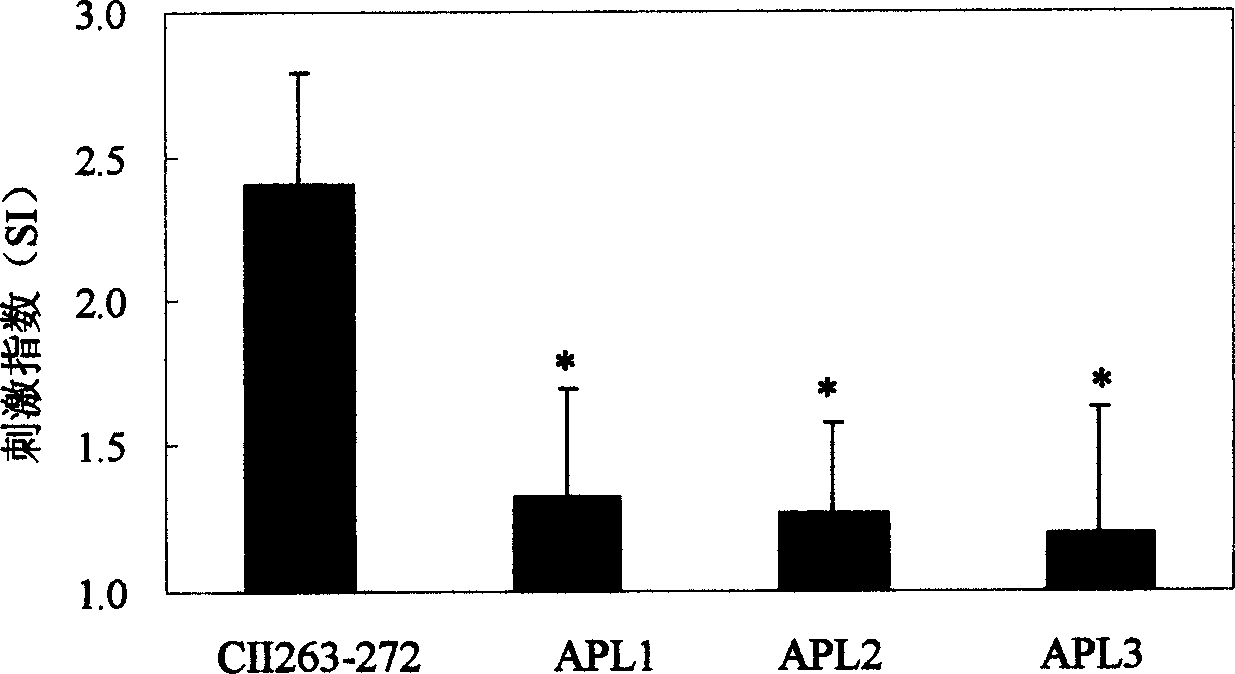
[0028] Example 3: Hyporesponsiveness of type II collagen allosteric peptides in peripheral blood T cells of RA patients with low or no T cell reactivity.
[0029] In an embodiment of the present invention, the reactivity of peripheral blood T cells of RA patients to three CII263-272 allosteric peptides was studied, and the production levels of Th1 type cytokines IL-2 and IFN-γ under the stimulation of allosteric peptides were detected. Variety.
[0030] Peripheral blood mononuclear cells (PBMCs) from RA patients were isolated by density gradient centrifugation and seeded in 96-well plates (2×10 5CII263-272 prototype peptide or three allosteric peptides (10 μg / ml) were added respectively, the positive control was phytohemagglutinin (10 μg / ml), and the negative control was 1640 culture solution. Make 3 replicate wells for each group, at 37°C, 5% CO 2 Cultivate under conditions for 5 days, add 1 μCi to each well 8 hours before the end of the culture 3 H-labeled thymidine ( 3 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com