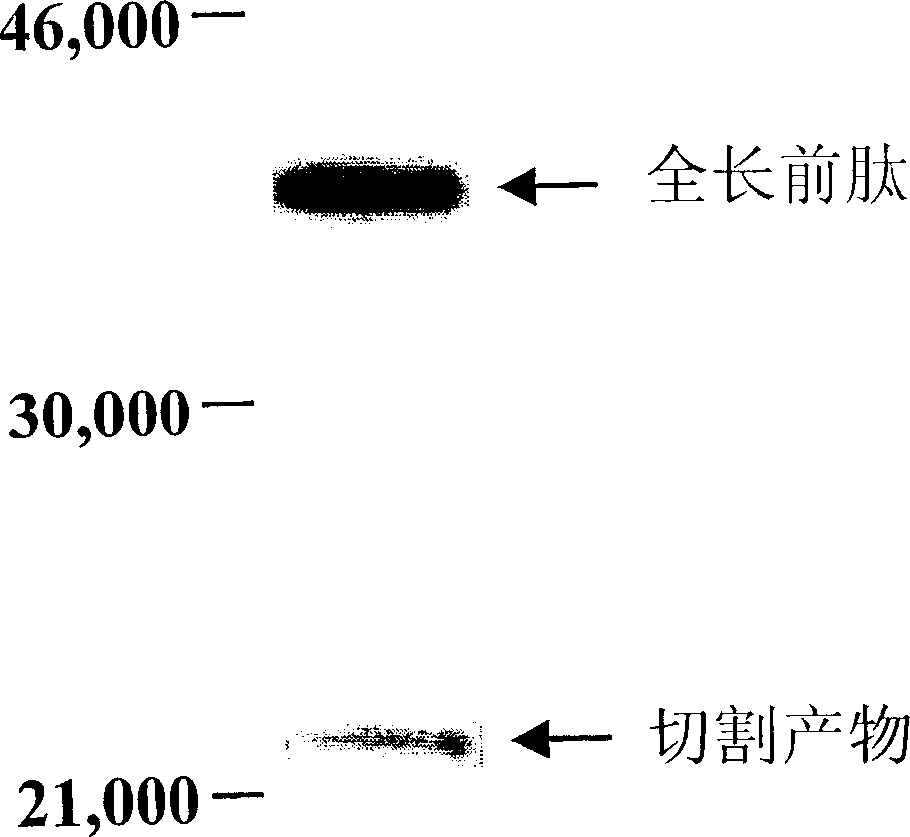Metalloprotease activation of myostatin, and methods of modulating myostatin activity
A technology of somatostatin and metalloprotease, applied in the field of metalloprotease activation of myostatin and regulating the activity of myostatin, can solve the problems of different and harmful effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
BMP-1 / TLD metalloproteinase family cleaves myostatin propeptide
[0087] This example demonstrates that members of the bone morphogenetic protein 1 / Tolloid (BMP-1 / TLD) family of metalloproteases cleave myostatin propeptide.
[0088] 500 ng of purified myostatin propeptide or purified latent myostatin complex containing propeptide and C-terminal dimer (Lee and McPherron, supra, 2001) was mixed with 100 ng of purified BMP-1 , mTLD, mTLL-1 or mTLL-2 (Scott et al., Devel. Biol. 213:283-300, 1999, which is incorporated herein by reference) were incubated overnight at 37°C. Reaction products were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by western blot analysis with antiserum against myostatin propeptide (Lee and McPherron, supra, 2001).
[0089] Isolated propeptide proteolytic cleavage products were detected in every reaction containing one of the four proteases, but not in control reactions containing no protease. Furthermor...
Embodiment 2
Cleavage of myostatin propeptide by metalloproteases activates latent myostatin
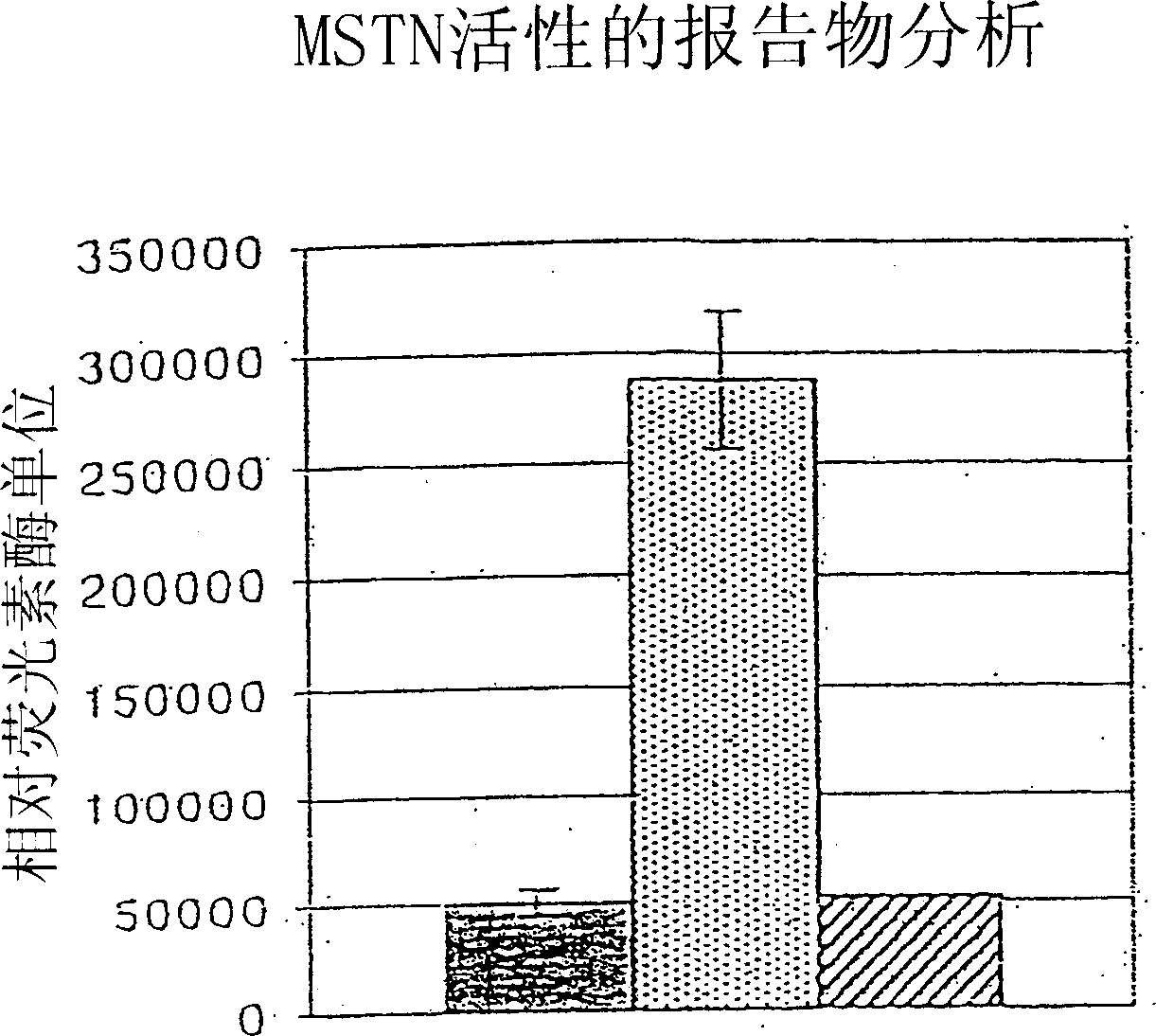
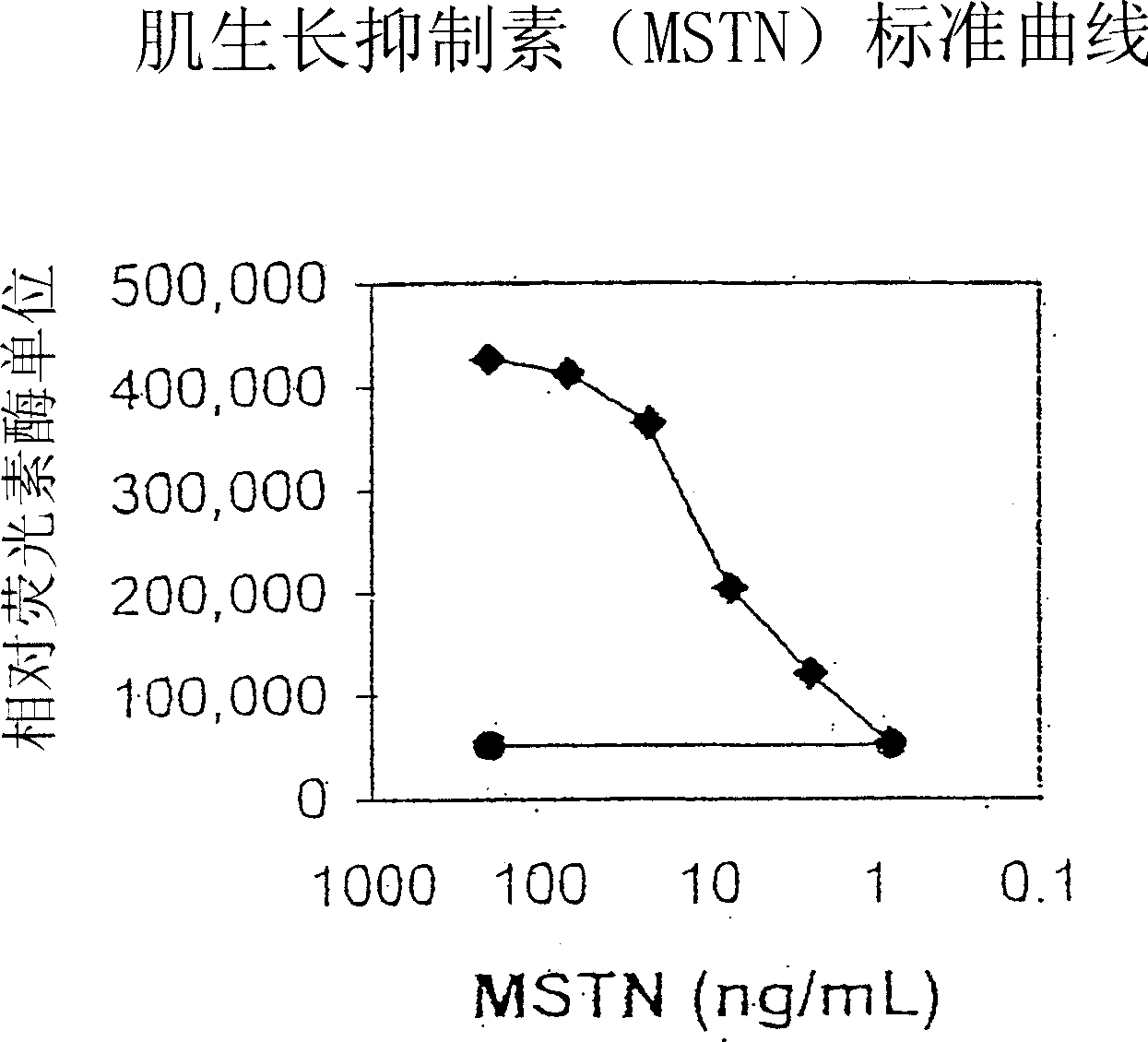
[0090] This example demonstrates that cleavage of myostatin propeptide by BMP-1 / TLD metalloproteases activates latent myostatin.
[0091] Purified myostatin propeptide and C-terminal dimer complexes were incubated with mTLL-1 and then examined using a reporter gene assay that specifically detects myostatin activity. with pGL3-(CAGA) 12 A204 rhabdomyosarcoma cells were transfected with a luciferase reporter construct comprising a luciferase coding sequence linked to a TGF-β-responsive CAGA sequence from the promoter of the TGF-β-inducible PAI-1 gene (Thies et al., supra, 2001). Transfected cells were contacted with untreated propeptide / C-terminal dimer complexes or with complexes that had been pre-incubated with mTLL-1. Incubation of complexes with mTLL-1 significantly increased the amount of luciferase activity detected in reporter cell assays, whereas complexes treated with mTLL-1 alone or wit...
Embodiment 3
Peptide substrates for members of the Tolloid family
[0093] Synthesize a series of peptides according to the sequence of myostatin propeptide, they are 10, 20, 30, 40 or 50 amino acid residues, each series includes three peptides, they all contain the corresponding BMP-1 / TLD The location of the cleavage site of the metalloprotease (in the wild-type peptide SEQ ID NOS: 9, 12, 15, 18 and 21, the amino acid residue "RD" is shown in bold, below). Peptides were synthesized in which the arginine residue at the P1 position immediately upstream of the cleavage site was changed to a glutamine residue (SEQ ID NOS: 10, 13, 16, 19 and 22; see bold), where Peptides were synthesized with aspartic acid changed to alanine at the P1' position immediately downstream of the cleavage site (SEQ ID NOS: 11, 14, 17, 20 and 23; see bold). The sequences of the peptides are listed below:
[0094] 50-mer (50-mer)
[0095]
KDVIRQLLPKAPPLRELIDQYDVQRDDSSDGSLEDDDYHATTETIIT
MPT (SEQ I...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com