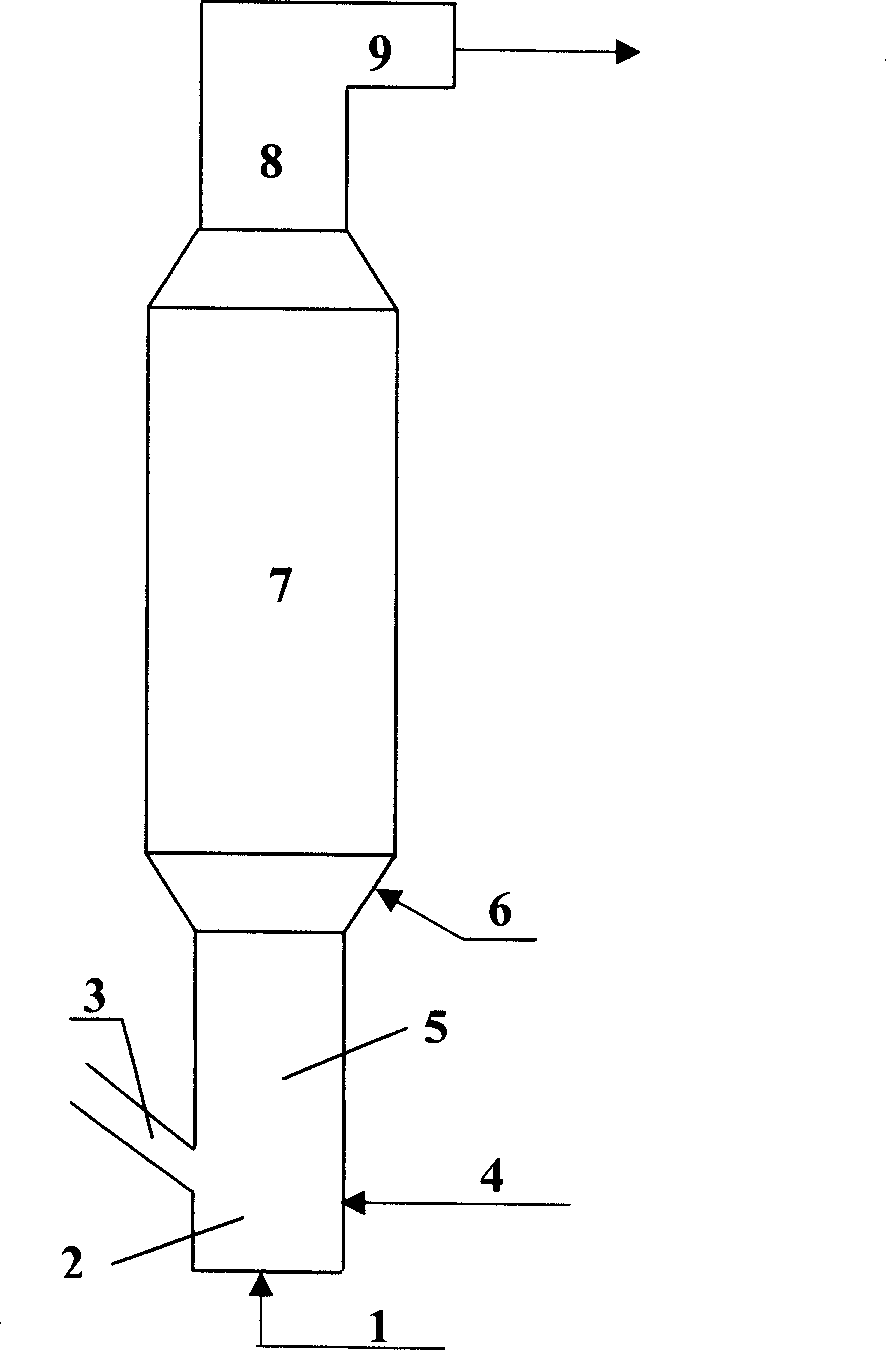
Method for catalytic cracking petroleum hydrocarbons
A technology of catalytic cracking and petroleum hydrocarbons, applied in catalytic cracking, cracking, petroleum industry, etc., can solve the problems of light oil components to be improved, and achieve the effects of decreasing olefin content, increasing heavy oil cracking capacity, and increasing yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0043] Preparation of the zinc and rare earth modified Y zeolite described in the present invention.
[0044] 60 kg of deionized water, 3 kg (NH 4 ) 2 SO 4 And 3 kilograms (dry basis) NaY zeolite (Qilu Petrochemical Company Catalyst Factory produces, NaY 2 O content 14% by mass (unit cell constant 2.473 nanometers) was added into a stirred tank, and ion exchange was performed at 90° C. for 0.5 hours. Filter, wash the solid with water, and roast at 600°C under 100% water vapor for 2 hours to produce HUSY zeolite. Re-slurry with 60 kg of decationized water in HUSY zeolite, followed by 2 liters of mixed rare earth chloride and ZnCl 2 The mixed solution, in which the zinc content is 60 grams as ZnO, and the rare earth content is as RE 2 o 3 It was calculated as 150 g, and ion exchange was performed at 90° C. for 0.5 hours. After filtration, the obtained solid product was washed with water, and then calcined in air at 600°C for 2 hours to obtain modified zeolite a. The chemi...
example 2
[0046] Preparation of the zinc and rare earth modified Y zeolite described in the present invention.
[0047] 60 kg of deionized water, 3 kg (NH 4 ) 2 SO 4 , 3 kg of NaY zeolite described in Example 1 and 2 liters of rare earth content in RE 2 o 3 100 g of the chlorinated mixed rare earth solution was added to a stirred tank, and ion exchange was performed at 90° C. for 0.5 hours. After filtering, the resulting solid product was washed with water, and then calcined at 600°C under 100% water vapor for 2 hours to produce REUSY zeolite. Slurry the resulting REUSY zeolite with 60 kg of decationized water, then add 2 liters of ZnCl with a zinc content of 60 g in terms of ZnO 2 The solution was subjected to ion exchange at 90°C for 0.5 hours. After filtration, the solid was washed with water, and calcined in air at 600°C for 2 hours to obtain modified zeolite b, whose chemical composition and main physical and chemical properties are shown in Table 1.
example 3
[0049] Preparation of the zinc and rare earth modified Y zeolite described in the present invention.
[0050] 60 kg of deionized water, 3 kg (NH 4 ) 2 SO 4 , 3 kilograms of the NaY zeolite described in Example 1, 2 liters of ZnCl with a zinc content of 100 grams in terms of ZnO 2 The solution was added to a stirred tank, and ion exchange was carried out at 90° C. for 0.5 hours. Filter, wash the solid with water, and bake at 600°C under 100% water vapor for 2 hours to make ZnUSY zeolite. The resulting ZnUSY zeolite was beaten with 60 kg of decationized water, and then 2 liters of rare earth content was added to RE 2 o 3 Calculate 150 grams of chlorinated mixed rare earth solution, carry out ion exchange at 90°C for 0.5 hours, filter, wash the solid matter with water, and roast in air at 600°C for 2 hours to obtain modified zeolite c. For its chemical composition and main physical and chemical properties, see Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com