Core-shell structured stationary phase for chiral ligand exchange chromatography and method for making same
A core-shell structure and chiral ligand technology, applied in the field of analytical chemistry, can solve the problems of low column efficiency, low column capacity and slow mass transfer of stationary phase, and achieve long service life, large column capacity and high selectivity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] (1) In 150 mL of anhydrous toluene, add 5 mL of vinyltrimethoxysilane and 5 g of silica gel (5 μm, amorphous), react at reflux temperature for 12 h, remove toluene and unreacted vinyltrimethoxysilane by suction filtration , the obtained solid was washed with toluene and methanol in turn, and dried to obtain modified silica gel.
[0020] (2) In 100 mL of anhydrous toluene, add 3 g of modified silica gel, 3 mL of glycidyl acrylate and 60 mg of azobisisobutyronitrile, react at 80°C for 24 hours, filter with suction, and dry the obtained solid by washing with toluene and methanol to obtain Core-shell structured silica gel 1.
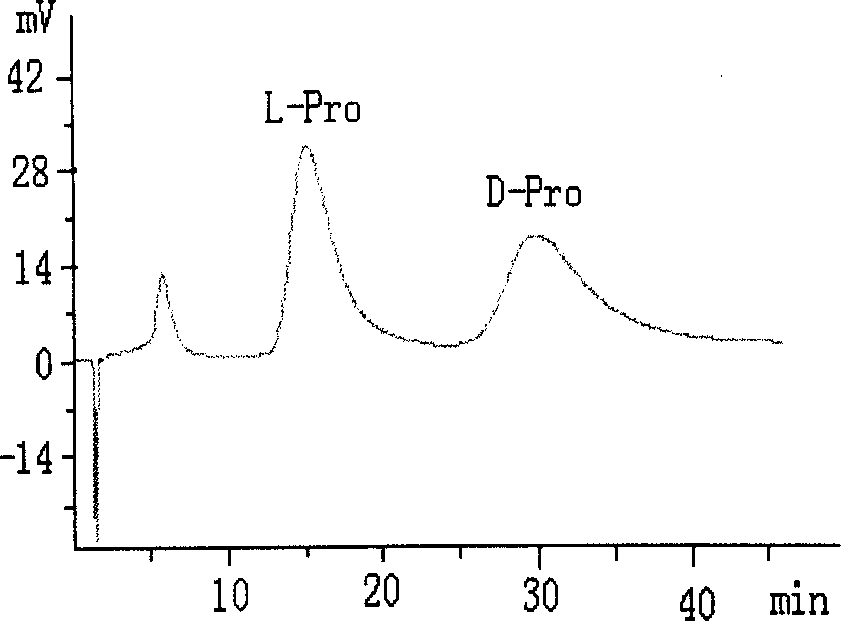
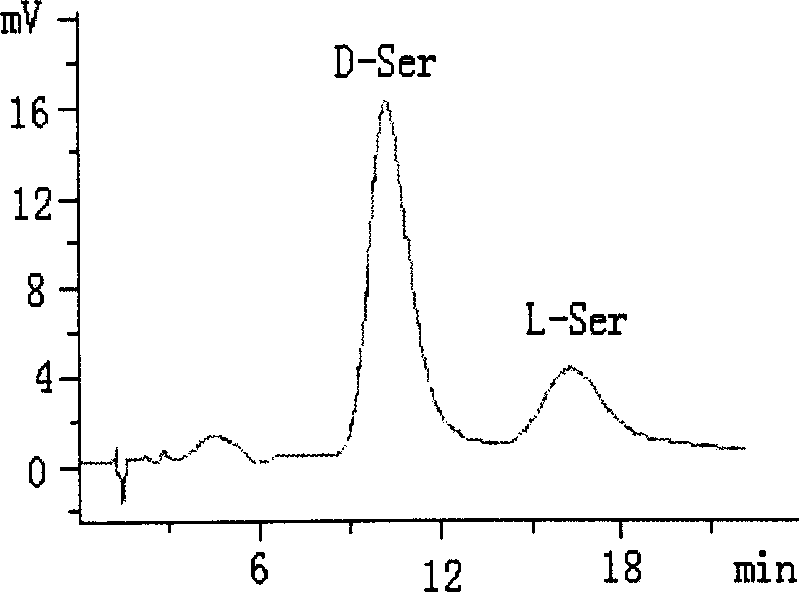
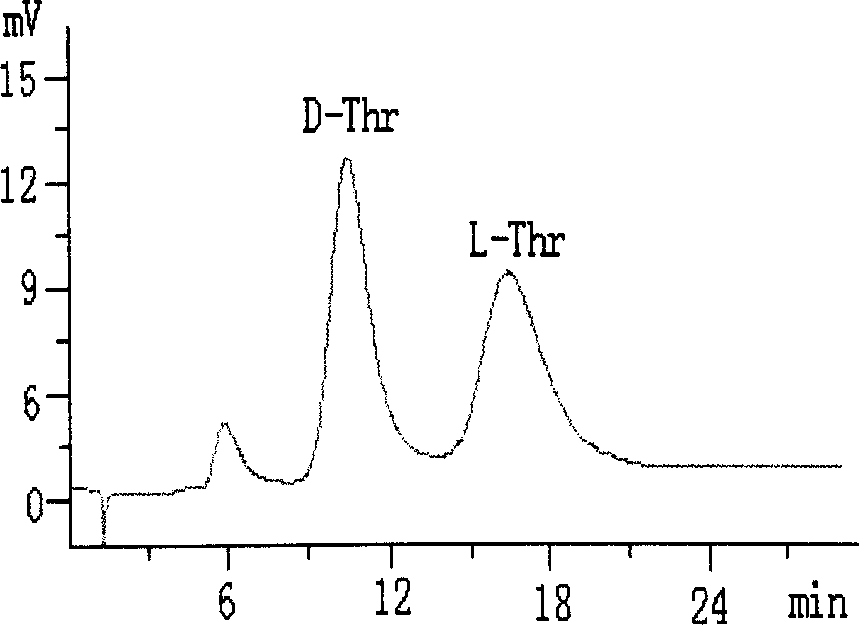
[0021] (3) In 100 mL of anhydrous methanol, add 2 g of core-shell structured silica gel 1 and 5 mmol of sodium L-proline, react at 40 °C for 24 h, and filter the obtained solid with methanol, methanol-water (v / v, 5 / 5), washed with water, and dried to obtain CS-LEP I chromatographic stationary phase. Elemental analysis: C 14.88%, H 2.32%, N 0.44%. ...
Embodiment 2
[0024] (1) Prepare modified silica gel according to step (1) of Example 1.
[0025] (2) In 100 mL of anhydrous toluene, add 3 g of modified silica gel, 1 mL of glycidyl acrylate and 20 mg (AIBN), react at 80°C for 2 h, then add 3 mL of glycidyl acrylate and 60 mg of azobisisobutyronitrile, The reaction was continued at 80° C. for 24 hours, and then filtered with suction. The obtained solid was washed with toluene and methanol to obtain silica gel 2 with a core-shell structure.
[0026] (3) The core-shell structure silica gel 2 is used instead of the core-shell structure silica gel 1, and the step (3) of Example 1 is carried out to obtain the CS-LEP II chiral stationary phase of the present invention. Elemental analysis: C 15.28%, H 2.30%, N 0.44%.
[0027] (4) The CS-LEP II stationary phase is used to replace the CS-LEP I phase, and according to the step (4) of Example 1, a chromatographic column and an equilibrium chromatographic column are prepared, and then used for the se...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com