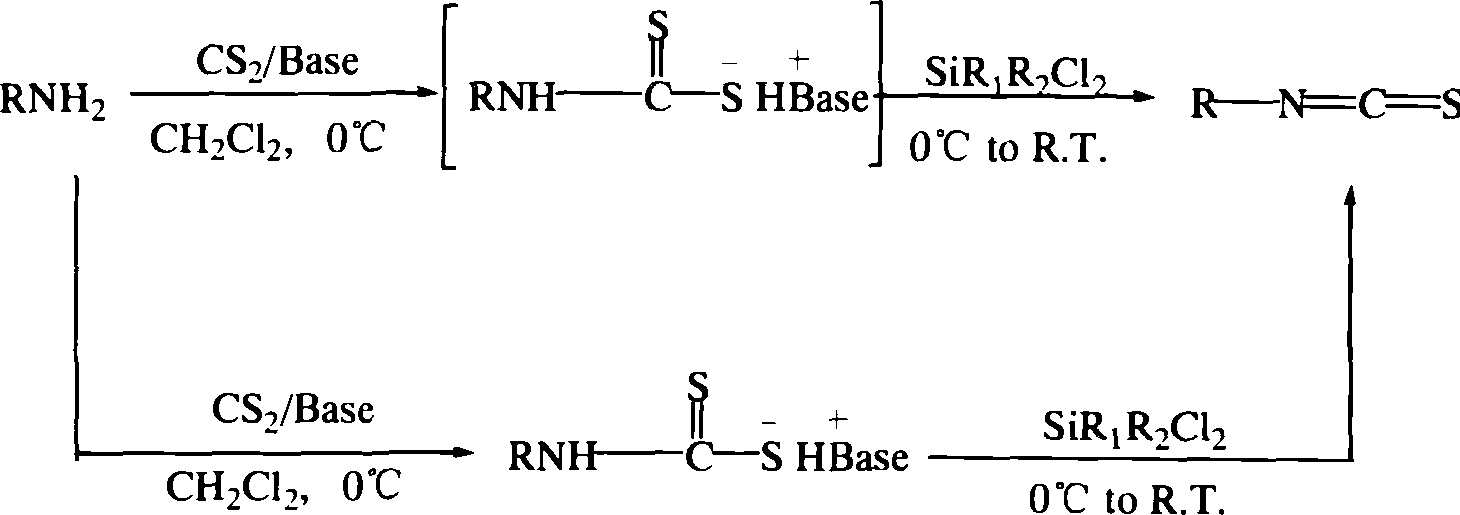
Method for synthesizing isorhodanate
A technology of isothiocyanate and synthesis method, applied in the direction of organic chemistry, etc., can solve the problems of difficulty in synthesis and purification of isocyanide, unfavorable industrialized large-scale production, etc., achieve great implementation value, social and economic benefits, and low production cost , the advanced effect of the process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1——the synthesis of p-nitrophenylisothiocyanate
[0023] In a 1000ml four-neck flask equipped with mechanical stirring, a dry constant pressure dropping funnel, a reflux condenser and a thermometer, add 1mol p-nitroaniline, 1mol triethylamine and 450ml tetrahydrofuran under nitrogen protection, start stirring, and At -10°C, slowly add carbon disulfide solution dissolved in 100 ml of tetrahydrofuran dropwise. After the addition and stirring for 5 hours, a large amount of solids precipitated, filtered with suction, and washed with a small amount of tetrahydrofuran. After drying, p-nitrobenzene dithioformate is obtained, and after recrystallization with solvents such as toluene, benzene, ethanol, methanol, ether, etc., high-purity p-nitrobenzene dithioformate is obtained.
[0024] Then above-mentioned 0.5mol of p-nitrobenzene dithioformate and a small amount of catalyst triethylamine are dissolved in 250ml of dichloromethane, and the dichloromethane solution tha...
Embodiment 2
[0025] Embodiment 2——the synthesis of p-chlorophenylisothiocyanate
[0026] In the 1000ml four-neck flask equipped with mechanical stirring, dry constant pressure dropping funnel, reflux condenser and thermometer, add 1mol p-chloroaniline, 1mol triethylenediamine 450ml dichloromethane under nitrogen protection, start stirring, and At 0-10°C, slowly add carbon disulfide solution dissolved in 100ml of dichloromethane dropwise. After adding and stirring for 5 hours, a large amount of solids precipitated, filtered with suction, and washed with a small amount of dichloromethane. After drying, p-chlorobenzenedithioformate was obtained.
[0027] Then above-mentioned 0.5mol of p-chlorobenzenedithioformate and a small amount of catalyst triethylamine were dissolved in 250ml of dichloromethane, and the dichloromethane solution of methylphenyldichlorosilane was slowly added dropwise. After adding, Slowly warm up to room temperature, then heat to reflux for 3-5 hours. After the reaction...
Embodiment 3
[0028] Embodiment 3——the synthesis of p-acetylphenylisothiocyanate
[0029] In a 1000ml four-neck flask equipped with mechanical stirring, a dry constant pressure dropping funnel, a reflux condenser and a thermometer, add 1mol p-acetylaniline, 1mol triethylenediamine, 450ml chloroform under nitrogen protection, and start stirring. Slowly add carbon disulfide solution dissolved in 100ml of dichloromethane dropwise at 0-10°C. After adding and stirring for 5 hours, a large amount of solids precipitated, filtered with suction, and washed with a small amount of chloroform. After drying, p-chlorobenzenedithioformate was obtained.
[0030] Then 0.5mol of the above-mentioned p-chlorobenzenedithioformate and a small amount of catalyst triethylamine were dissolved in 250ml of dichloromethane, and the dichloromethane solution of dimethyldichlorosilane was slowly added dropwise. The temperature was raised to room temperature, and then heated to reflux for 3-5 hours. After the reaction w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com