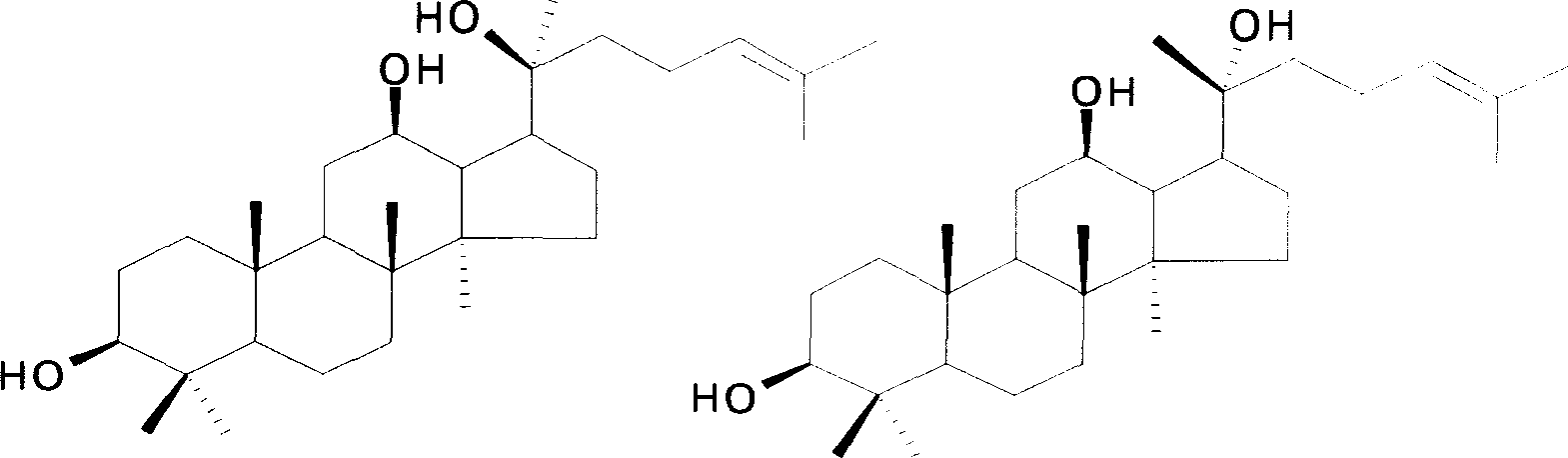
Use of 20(S)-protopanoxadiol in preparation of anti-bowelcancer medicine
A protopanaxadiol and drug technology, applied in the field of medicine, can solve the problems of not improving the 5-year survival rate, high toxicity and side effects of chemotherapy, and short effective time limit, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] 18kg of Panax notoginseng (specification: countless heads, purchased from Yunnan) is crushed into powder (100-200 mesh), soaked in 30kg of 95% ethanol for two days, filtered, the filtrate is concentrated to obtain Panax notoginseng ethanol extract, and the ethanol is recovered and reused to soak the filter residue Six times, finally accumulatively obtained 3.37kg of Panax notoginseng ethanol extract, dissolved it in water, extracted three times with petroleum ether, took the water phase and extracted four times with n-butanol, concentrated the n-butanol layer, and obtained Panax notoginseng total saponins n-butyl Alcoholic extract 1.78kg.
[0089] Get total saponin extract 100g and dissolve in 1300ml n-butanol, heat, stir, add sodium ethylate (chemically pure, purity: 80%) 132.6g (1.56mol, concentration: 1.2mol / L), feed oxygen, 90 ℃ react After 65 hours, the reaction was over. The reaction solution was cooled to room temperature, washed with water saturated with n-buta...
Embodiment 2
[0091] Get 100g of Panax notoginseng total saponins extract (prepared in Example 1) and dissolve in 1500ml n-amyl alcohol, heat, stir, add sodium n-butoxide (chemically pure, purity: 80%) 150g (1.56mol, concentration: 1.04 mol / L), pass compressed air, react at 100°C for 60 hours, and the reaction ends. The reaction solution was cooled to room temperature, washed with water saturated with n-butanol, the n-butanol layer was concentrated and dissolved in water, extracted with ethyl acetate, the ethyl acetate layer was washed with water, and dried. After concentration, it was purified by silica gel column chromatography [cyclohexane:ethyl acetate=10:1-1:1 (V / V) gradient elution] to obtain 8 g of protopanaxadiol (A2), with a purity of 97% as determined by HPLC. %;
Embodiment 3
[0093] Notoginsenoside (content: calculated as ginsenoside Rb3, 91.9%; purchased from Yunnan) 1000g was dissolved in 14L n-butanol, heated, stirred, and sodium ethylate (chemically pure, purity: 80%) 1190g (14.0mol, Concentration: 1.0mol / L), pass oxygen, react at 110°C for 55 hours, and the reaction ends. The reaction solution was cooled to room temperature, washed with water saturated with n-butanol, the n-butanol layer was concentrated and dissolved in water, extracted with ethyl acetate, and the ethyl acetate extract was washed with water, dried, and evaporated to dryness. After purification by silica gel column chromatography [petroleum ether: ethyl acetate = 9:1-2:1 (V / V) gradient elution], 181.6 g of protopanaxadiol (A2) was obtained, and the purity determined by HPLC was 99.3%. Its physicochemical data are consistent with literature values: J. Asakawa et al, Tetrahedron, 1977, 33, 1935-1939; Nagai, M. et al, Chem. Pharm. Bull, 1972, 20(6), 1212-1216.
[0094] The physi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com