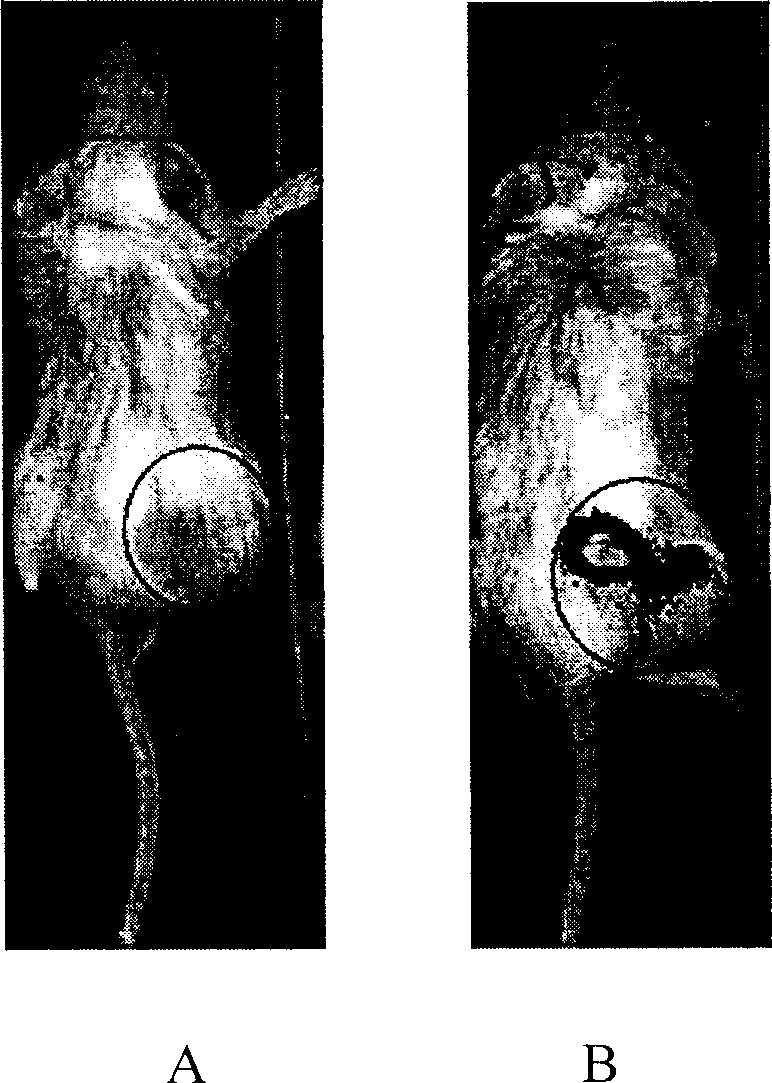Application of expression TRAIL protein colon bacillus for preparing medicine to treat tumor
A technology for the treatment of Escherichia coli and tumors, which is applied in the direction of anti-tumor drugs, gene therapy, drug combinations, etc., to achieve broad market prospects and solve the effects of toxic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Establishment of animal tumor model and metastatic tumor model
[0039] 1. Materials:
[0040] (1) Cells, bacteria and culture medium
[0041] Tumor cells B16-F10-luc-G5, MCF-7-luc-F5, BEL7405-luc and SMMC7721-luc were purchased from Xenogen Company, PC-3, C6, MB-49 and HT1080 were purchased from ATCC Company, E.coli DH5α , BL21 and JM109 are commercially available and kept in this laboratory. Cell culture medium: RPMI-1640, DMEM, and fetal bovine serum are products of Sigma Company. Bacterial culture medium: peptone and yeast extract of each component in LB were purchased from OXOID Company, and sodium chloride was domestic analytical grade.
[0042] (2) Plasmids, vectors and libraries
[0043] The pXen-1 (luxABCDE) plasmid was purchased from Xenogen Company, pGEX-KG, pET-28a(+) and pBV220 were purchased from the market and kept in our laboratory, and the human breast cDNA library was purchased from Clotech Company.
[0044] (3) Experimental anim...
Embodiment 2
[0064] Example 2 Construction and Detection of Luminescent Bacteria
[0065] 1. Materials
[0066] Same as Example 1.
[0067] 2. Methods
[0068] (1) Preparation of luminescent bacteria
[0069] Bacterial medium LB was prepared as usual, and competent cells such as Escherichia coli DH5α, BL21, and JM109 were prepared as usual.
[0070] The pXen-1 (luxABCDE) was transformed into Escherichia coli DH5α, BL21 and JM109 according to the conventional method, and the luminescence and efficiency of the clones were observed by IVIS imaging system after plating.
[0071] (2) Treatment of bacteria before the experiment
[0072] ① One day before injecting bacteria into mice, pick monoclonal colonies with higher luminescence efficiency and culture them in LB with Amp resistance overnight, transfer the bacteria at 1:100 the next day, and continue to shake until logarithmic growth The middle and late stage is OD600=0.6~1.0.
[0073] ②Press 1OD=8×10 8 Calculate the amount of b...
Embodiment 3
[0079] Example 3 Study on the distribution characteristics and tumor localization characteristics of bacteria in animals
[0080] 1. Materials:
[0081] Same as Example 1.
[0082] 2. Methods and Results
[0083] (1) Characteristics of bacteria distribution in normal mice
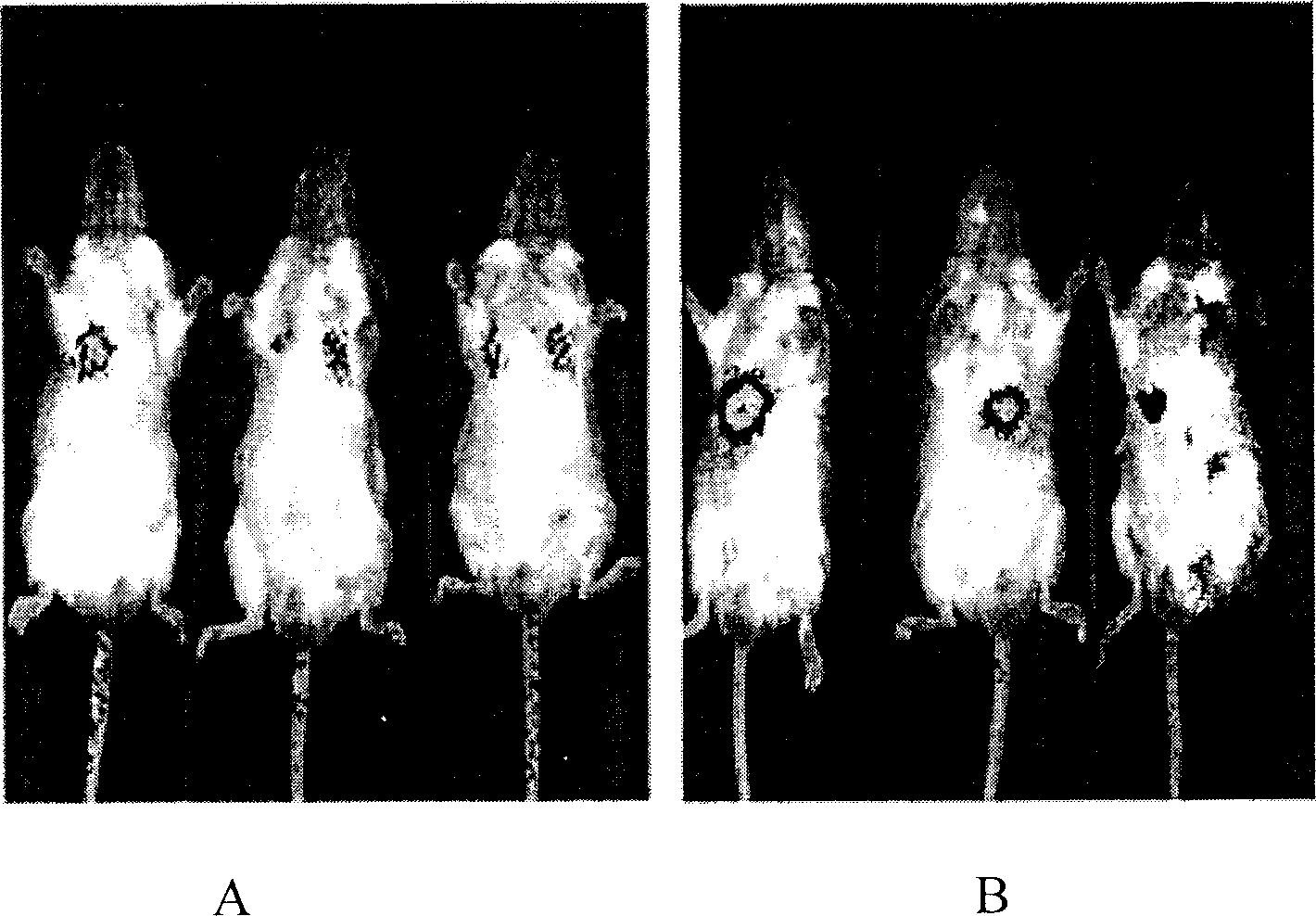
[0084] 2 x 10 intravenously 9 cfu / 100μl / mouse or 1×10 9 Within 10 to 20 minutes of cfu / 100μl / mouse bacteria in normal mice, the bacteria were distributed in the lungs, and then the bacteria gradually accumulated in the liver and localized in the liver (see appendix). figure 1 ). Most mice cannot tolerate greater than 1×10 9 Cfu / 100μl / mouse dose of bacteria, most mice died on the second day after injection, autopsy results showed that bacteria mainly accumulated in the lungs and livers of mice. The bacteria are only distributed in the liver. Intravenous 1×10 8 The mice with cfu / 100μl / mouse bacteria could all survive, and the distribution of bacteria was finally distributed only to the liver.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com