Process for iohexol manufacture
A technology of iohexol and reaction, applied in the field of non-ionic X-CT contrast agent, can solve problems such as difficult to remove, large product loss, etc., achieve prevention of O-alkylation by-products, low production cost, and easy operation safe effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
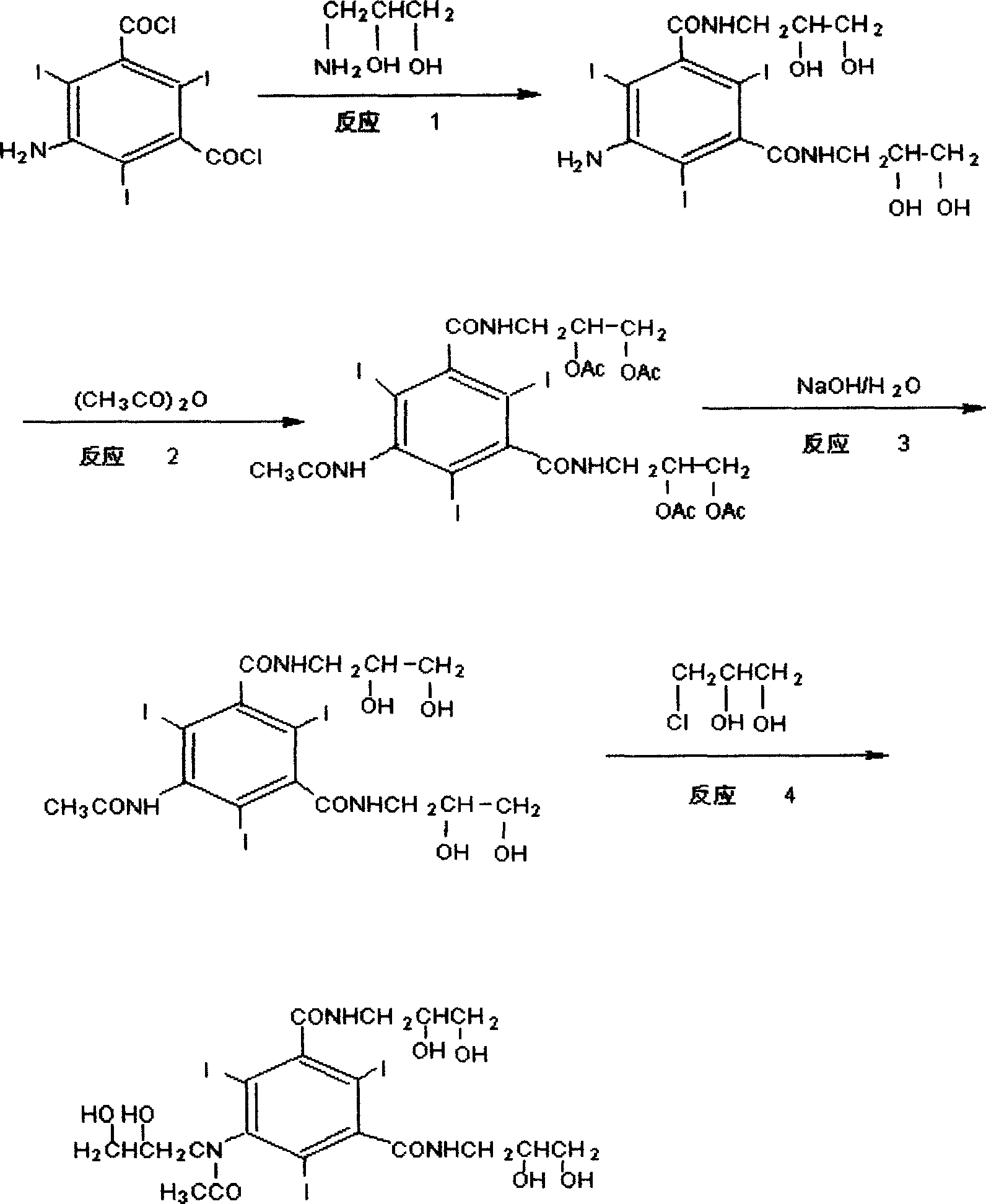
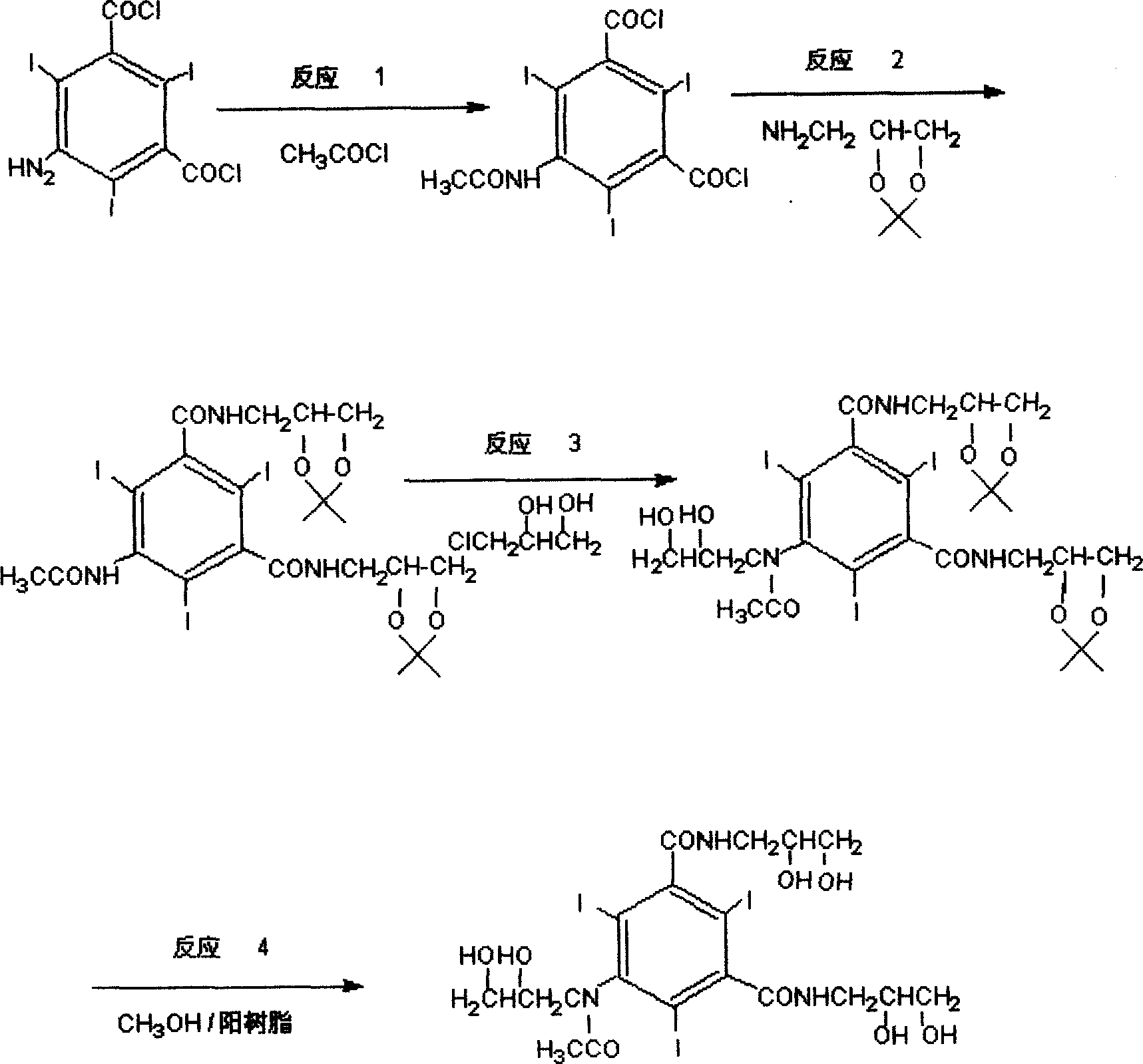
[0034] Reaction 1: Preparation of 5-acetylamino-2,4,6-triiodo-1,3-benzoyl chloride
[0035] 120 g of 5-amino-2,4,6-triiodo-1,3 benzoyl chloride and 360 ml of dioxane were heated to 40°C-50°C to dissolve completely, and then a small amount of catalyst was added. 17.4 g of acetyl chloride was added dropwise while keeping warm. After the drop was completed, the stirring was continued for 30 minutes, and the temperature was raised to reflux for 4 hours. Part of the solvent was evaporated under reduced pressure, and 800 ml of dichloromethane was added. A solid precipitated overnight, filtered, and dried. 116.8 g of the product was obtained, yield 91%, melting point: 91°C
[0036] Reaction 2: Preparation of 5-acetylamino-2,4,6-triiodo-1,3-aminopropylketal pyridene benzamide
[0037] 128g of 5-acetylamino-2,4,6-triiodo-1,3-benzoyl chloride, 320ml of DMAC, stirred and dissolved, then 60ml of triethylamine was added, and 58g of aminopropyl ketal fork was added dropwise at a temperat...
Embodiment 2
[0043] Put 5-amino-2,4,6-triiodo-1,3 benzoyl chloride (120g) and dioxane (360ml) into a three-neck flask, heat up to 40-50°C and dissolve completely, then add a little catalyst. Acetyl chloride (17.4 g) was added dropwise while keeping warm. After the drop was complete, stirring was continued for 30 minutes, and the temperature was raised to reflux for 4 hours. Part of the solvent was evaporated under reduced pressure, and dichloromethane (800ml) was added. A solid precipitated overnight, filtered, and dried. The product (116.8g) was obtained, yield 91%, melting point: 91°C
[0044] Stir 5-acetylamino-2,4,6-triiodo-1,3-benzoyl chloride, DMA, and triethylamine evenly, cool to 0-5°C, add 3-aminopropaneketal dropwise, and control the dropwise The acceleration is 15-20d / min, the dropping temperature is 0-5°C, the stirring reaction is continued for 1h after the dropping, and the solvent is recovered by distillation under reduced pressure. Purified water was added, and after stir...
Embodiment 3
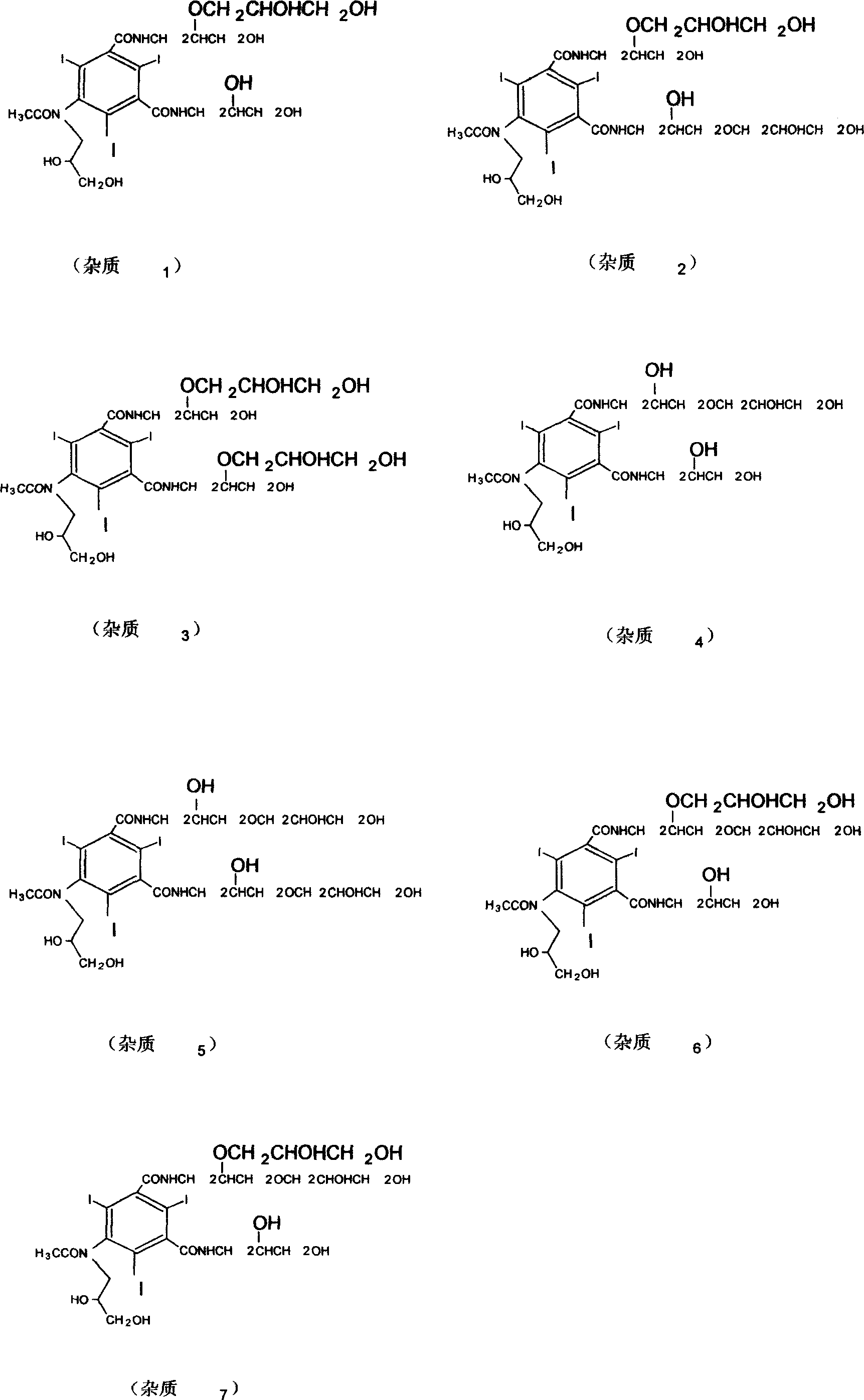
[0045] Embodiment three: this embodiment reaction 1, reaction 2 are the same as example two, the difference is that in reaction 3, 3-chloro-1,2-propanediol (9.9g) is replaced by 3-chloro-1,2-propanediol Ketoylidene (13.5g) was hydrolyzed after complete reaction to obtain iohexol, and then was removed with isopropanol, frozen and crystallized, overnight, and suction filtered. The product (72.4 g) was obtained in 85% yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com