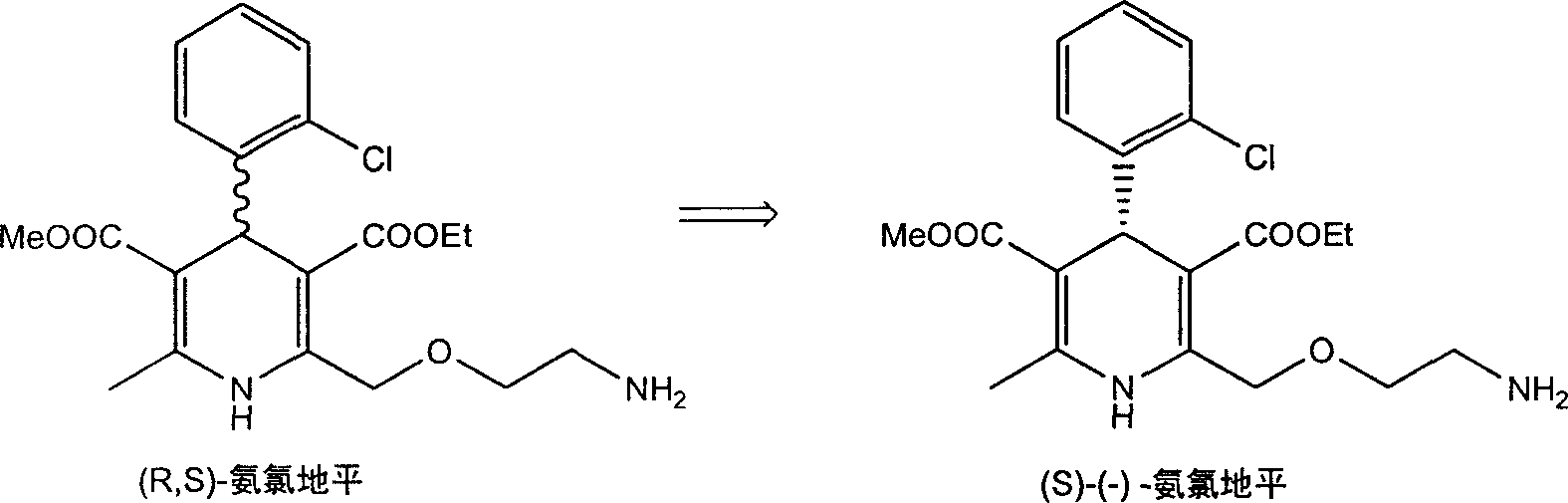
Method for splitting Amlodipine
A technology of amlodipine and methylpyrrolidone, which is applied in the direction of organic chemistry, can solve the problems of high price of deuterated dimethyl sulfoxide and lack of industrial application prospects, and achieves cheap and easy-to-obtain reagents, simple methods, and split The effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1 is prepared by (R, S)-amlodipine (S)-(-)-amlodipine-semi-D-tartaric acid-single-N-methylpyrrolidone complex and (R)-(+)- Amlodipine-Semi-D-Tartrate-Mono-N-Methylpyrrolidone Complex
[0020] In a 250 ml egg-shaped bottle, 57 g of (R,S)-amlodipine was dissolved in 200 ml of N-methylpyrrolidone. A solution of 10.5 grams of D-tartaric acid dissolved in 200 milliliters of N-methylpyrrolidone was added dropwise into the reaction system, and left to react at room temperature for 1 hour. Add a small amount of (S)-(-)-amlodipine-hemi-D-tartrate as a seed crystal, and continue static crystallization at room temperature for 12 hours. Filter under reduced pressure, wash the obtained white solid with 50 ml of acetone, and dry under reduced pressure in vacuo for 10 hours. 26 g (80% of theory) of (S)-(-)-amlodipine-hemi-D-tartaric acid-mono-N-methylpyrrolidone complex were obtained.
[0021] Compound 3-30
[0022] Molecular formula: C 27 h 38 o 7 ClN 3 o 9
[00...
Embodiment 2
[0039] Example 2 Preparation of (R)-(+)-amlodipine-semi-L-tartaric acid-single-N-methylpyrrolidone complex and (S)-(-)-by (R, S)-amlodipine Amlodipine-Semi-L-Tartrate-Mono-N-Methylpyrrolidone Complex
[0040] In a 250 ml egg-shaped bottle, 11.84 g of (R,S)-amlodipine was dissolved in 50 ml of N-methylpyrrolidone. A solution of 4.2 g of L-tartaric acid dissolved in 40 ml of N-methylpyrrolidone was added dropwise into the reaction system, and stirred at room temperature for 5 hours. A small amount of (R)-(+)-amlodipine-hemi-L-tartrate was added as a seed crystal, and stirring and crystallization were continued at room temperature for 12 hours. Filter under reduced pressure, wash the obtained white solid with 50 ml of acetone, and dry under reduced pressure in vacuo for 10 hours. This gave 4.62 g (70% of theory) of (R)-(+)-amlodipine-semi-L-tartaric acid-mono-N-methylpyrrolidone complex.
[0041] Compound 3-30
[0042] Molecular formula: C 27 h 38 o 7 ClN 3 o 9
[0043]...
Embodiment 3
[0057] Example 3 Preparation of (S)-(-)-amlodipine (alkaline hydrolysis) by (S)-(-)-amlodipine-half-D-tartaric acid-mono-N-methylpyrrolidone complex
[0058] 5 g of (S)-(-)-amlodipine-semi-D-tartaric acid-mono-N-methylpyrrolidone complex were stirred with 15 ml of 2N aqueous sodium hydroxide solution and 15 ml of dichloromethane for 40 minutes. Oil and water were separated, the organic layer was washed twice with water, dried over anhydrous sodium sulfate, concentrated under reduced pressure, replaced with petroleum ether and stirred to crystallize to obtain a white solid, filtered under reduced pressure, and dried in vacuo to obtain 3.2 g of (S)-(-)-amlodipine ( 88% of theoretical yield).
[0059] Molecular formula: C 20 h 25 o 7 ClN 2 o 5
[0060] m.p.110-111℃
[0061] [α] D =-31.8° (c=1.25, MeOH)
[0062] Optical purity 99.7% d.e. (chiral HPLC)
[0063] 1 H NMR (300Hz, CDCl 3 )δ: 7.96(s, 1H), 7.31(d, 1H, J=7.8Hz), 7.15(d, 1H, J=7.8Hz), 7.06(t, 1H, J=7.5Hz), 6.97...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com